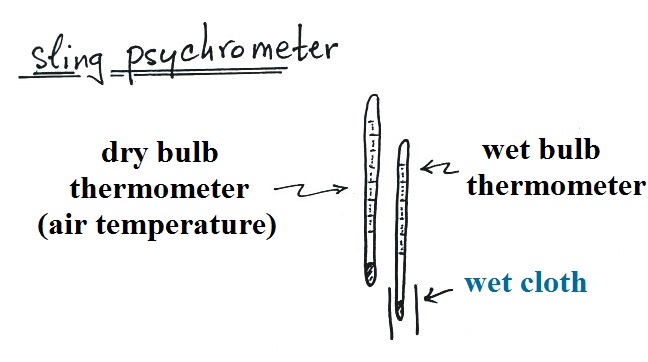

 |
 |
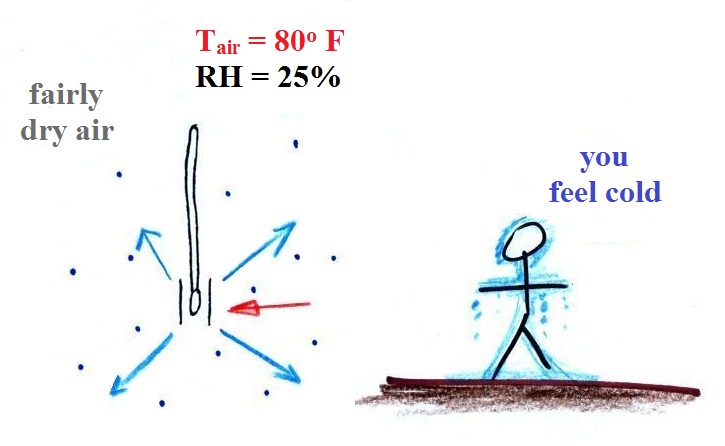

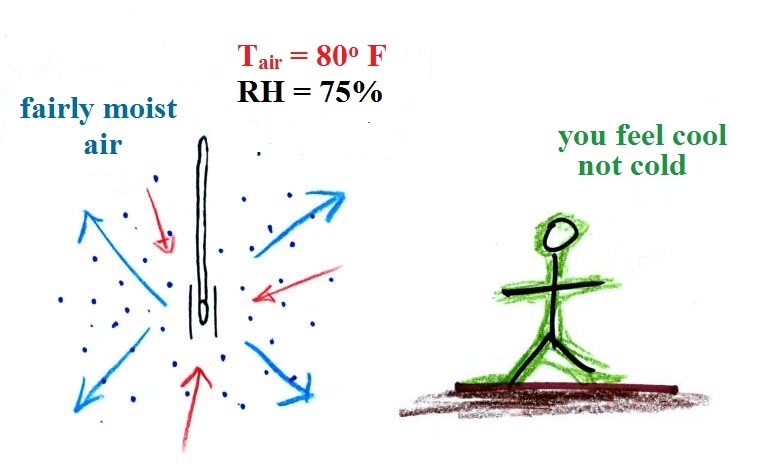

|
Here's
the summary
|
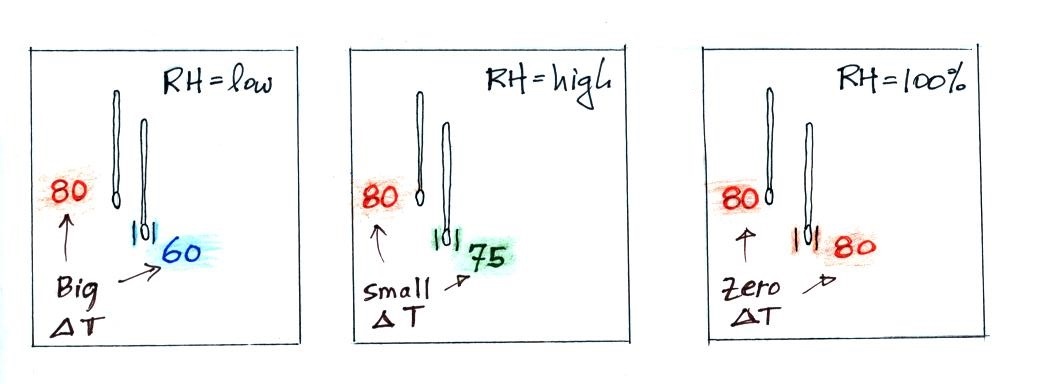
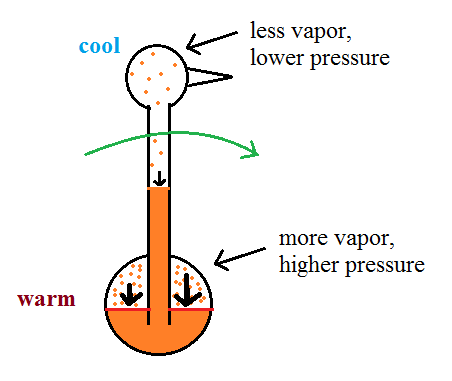
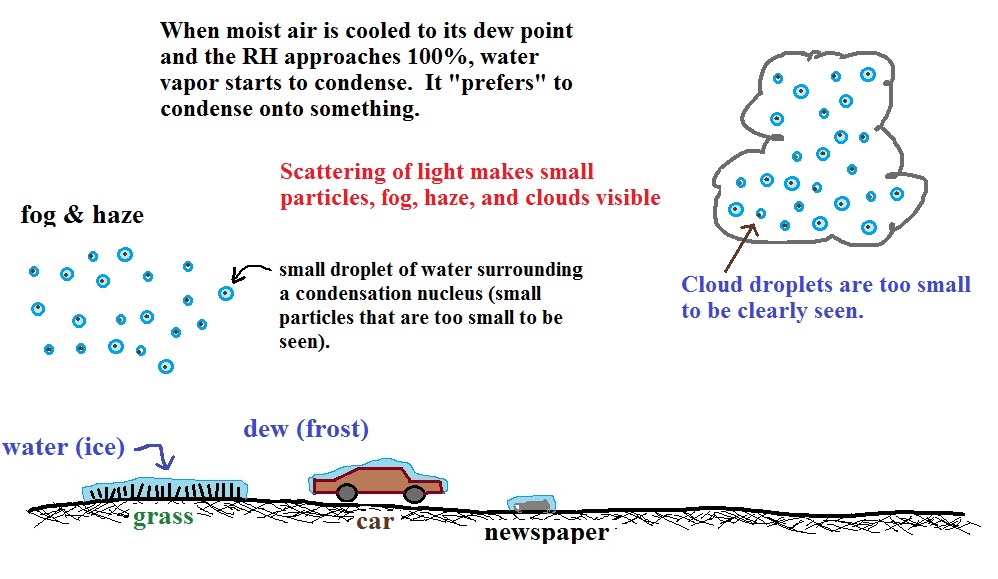
| it is much
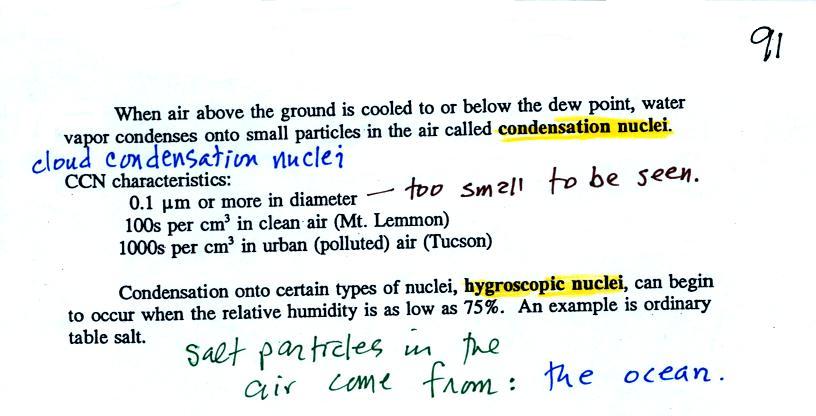
easier for water vapor to condense onto small particles called condensation nuclei |
it would be
much harder for
water vapor to just condense and form small droplets of pure water |
When
the air is saturated with water vapor (the
relative humidity is 100%) the rates of
evaporation and condensation above a flat
surface of water will be equal.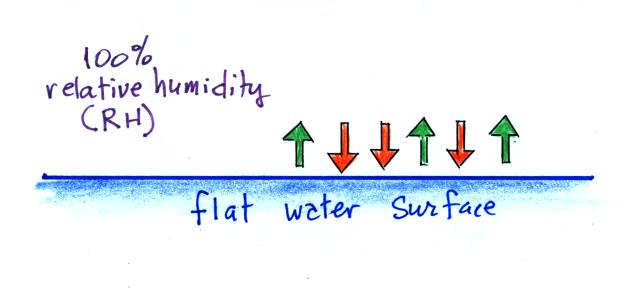 It's hard for water vapor to condense and form a small droplet of water because small droplets evaporate at a very high rate. This is known as the curvature effect and is illustrated below. 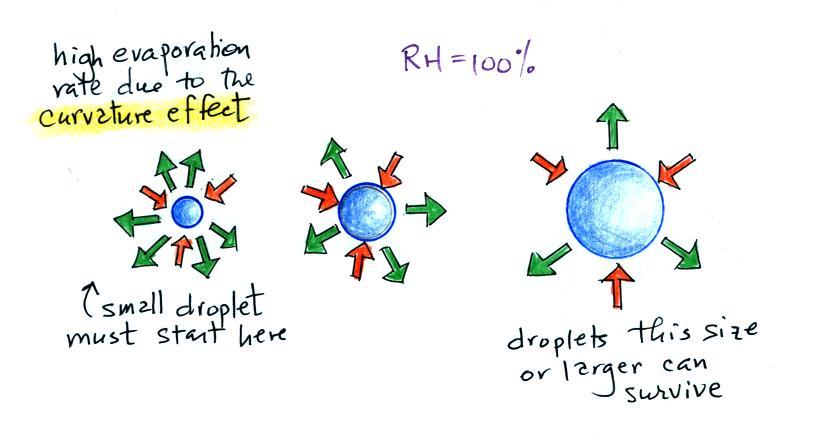 The surface of the smallest droplet above at left has the most curvature and the highest rate of evaporation (6 arrows). If a small droplet like this were to form, it wouldn't stay around very long. With it's high rate of evaporation it would quickly evaporate away and disappear. The middle droplet is larger and would stick around a little longer because it does not evaporate as quickly. But it too would eventually disappear. The drop on the right is large enough that curvature no longer has an effect. This drop has an evaporation rate (3 arrows) that is the same as would be found over a flat surface of water. A droplet like this could survive, but the question is how could it get this big without going through the smaller sizes with their high rates of evaporation. A droplet must somehow reach a critical size before it will be in equilibrium with its surroundings. Particles in the air, cloud condensation nuclei (CCN), make it much easier for cloud droplets to form. The figure below explains why. 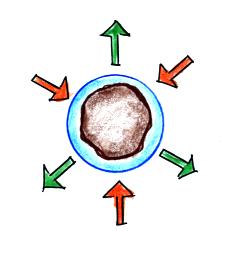 There are always lots of CCN (cloud condensation nuclei in the air) so this isn't an impediment to cloud formation. |
To understand how
condensation onto particles can begin even before the RH
has reached 100% we first need to learn about the solute
effect
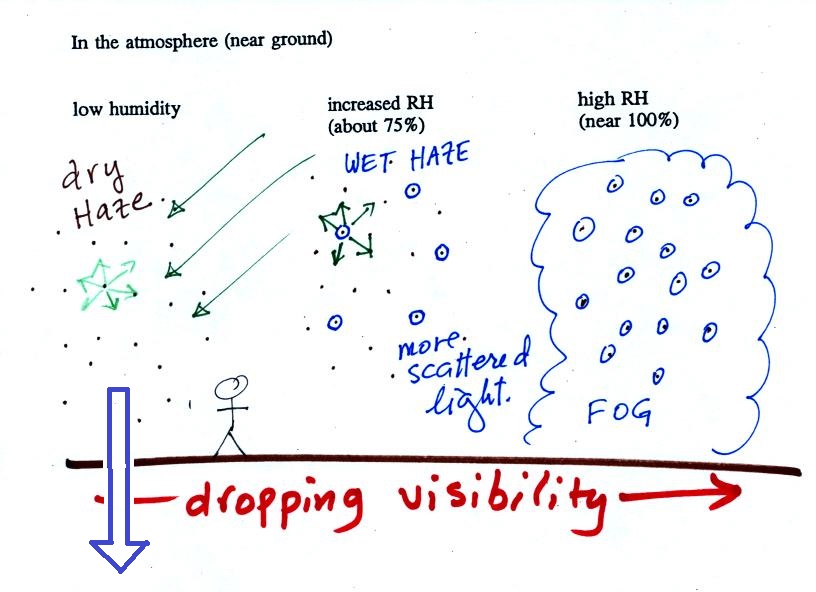
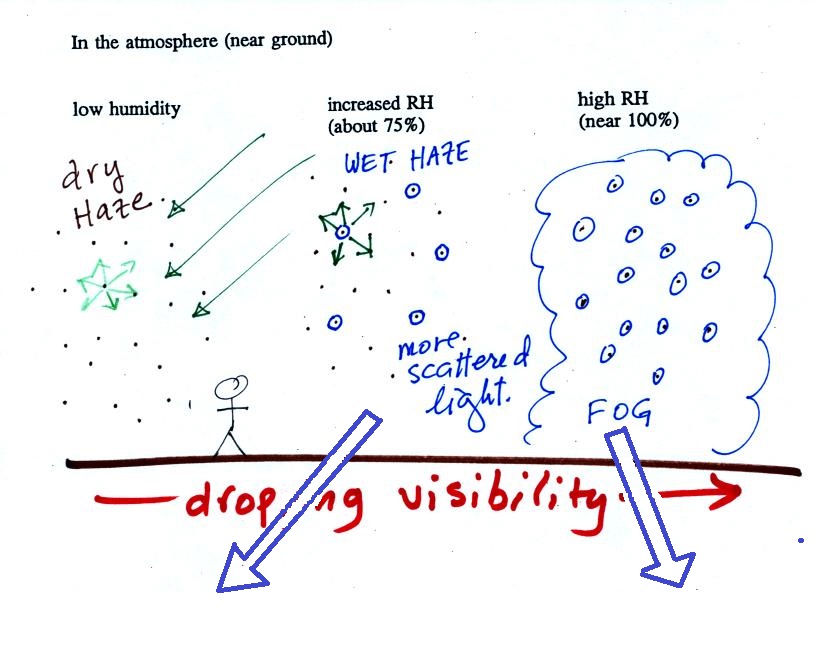
The next figure compares solution droplets that form when the RH is 100% (left figure) and when the RH is less than 100%.
The solution droplet will grow in the RH=100% environment at left. You can tell the RH is less than 100% in the figure at right because there are now only 2 arrows of evaporation. But because the solution droplet only has 2 arrows of evaporation it can form and be in equilibrium in this environment. |

 |
 |
| Thin fog
(perhaps even wet haze) with pretty good visibility (source of the image) |
Thick fog (visibility was less than 500 feet) (source of the image) |
| Pictures of fog like
we sometimes get in Tucson (maybe once a year). The
picture at left is looking east from my house and was taken
early in the morning at the start of the spring semester in
2015. The picture at right is the view to the
west. Visibility was perhaps 1/4 mile. |
|