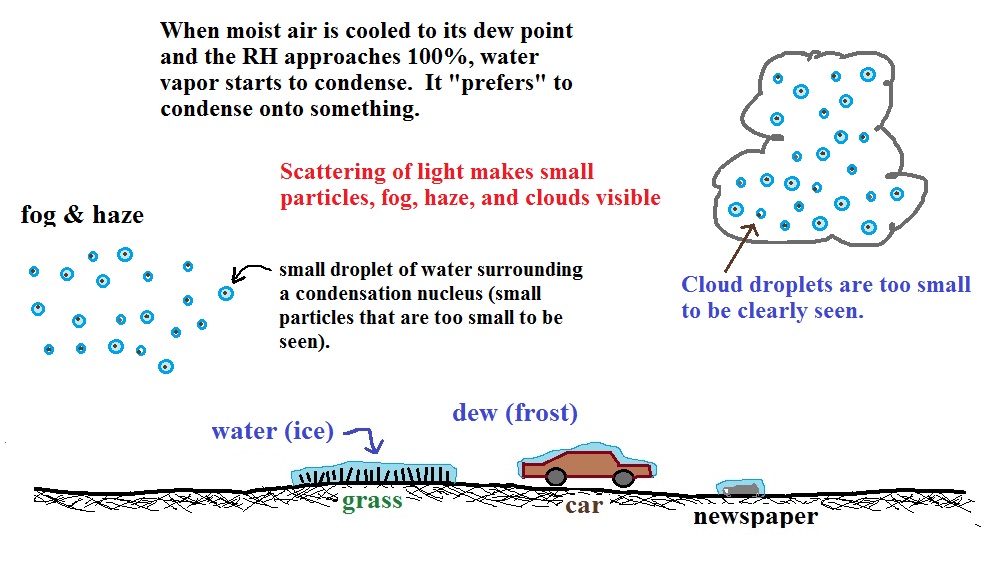
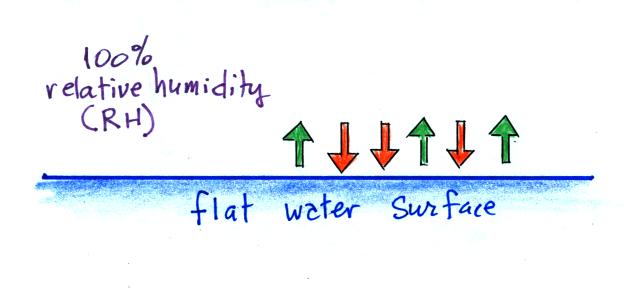
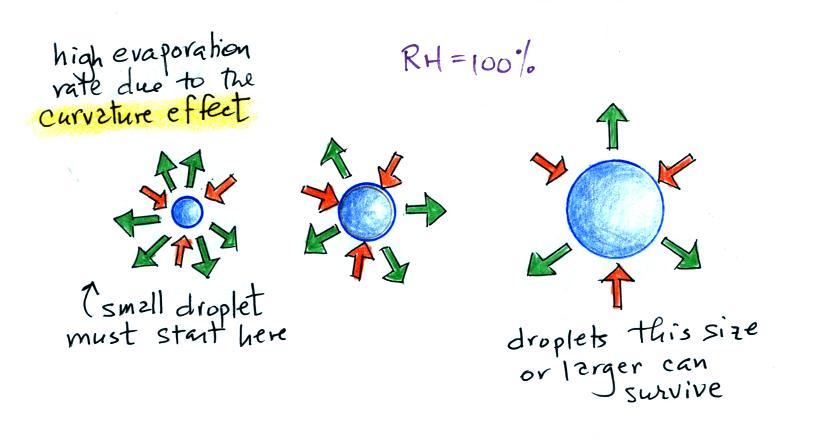
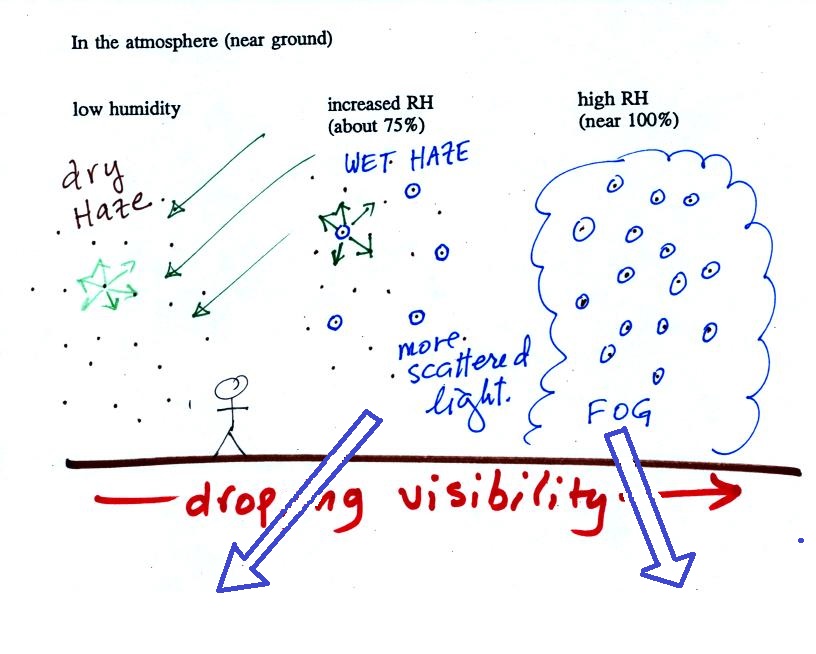
| Tair = ?
|
r = 10.5 g/kg |
| RH = 50% | Td = ? |
You're given the the mixing ratio = 10.5 g/kg and a relative humidity of 50%. You need to figure out the air temperature and the dew point temperature. Give it a try before you have a look at the step by step solution below:
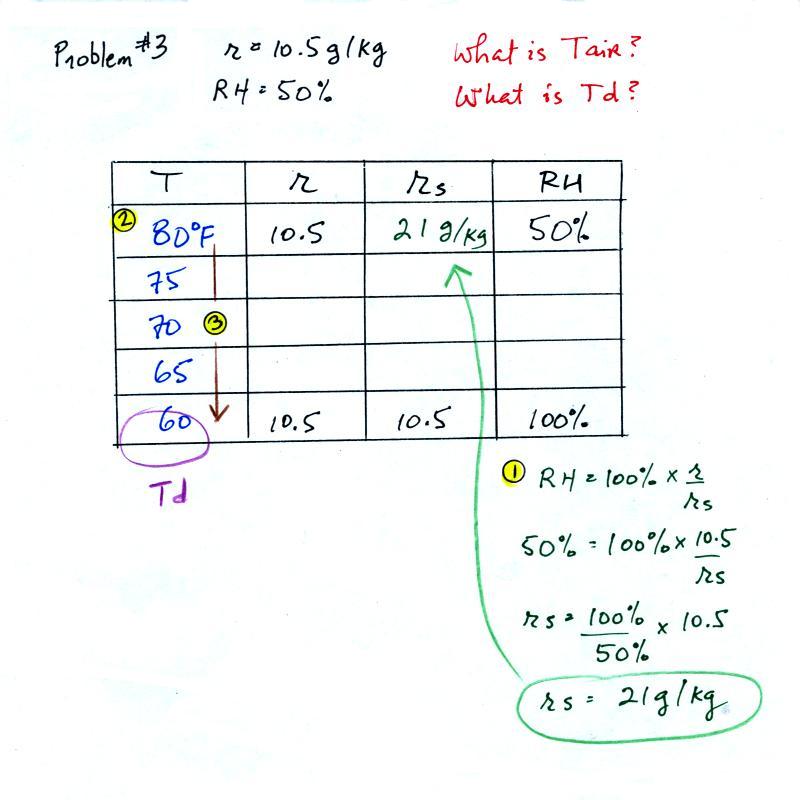
(1) The air contains 10.5 g/kg of water vapor. This is 50% (half) of what the air could potentially hold. So the air's capacity, the saturation mixing ratio must be 21 g/kg (you can either do this in your head or use the RH equation following the steps shown above).
(2) Once you know the saturation mixing ratio you can look up the air temperature in a table (80 F air has a saturation mixing ratio of 21 g/kg)
(3) Then you imagine cooling the air until the RH becomes 100%. This occurs at 60 F. The dew point is 60 F
Humidity example problem #4
One of the "jobs" of the dew point, to give you an idea of the actual amount of water vapor in the air, is the same as the mixing ratio. The next problem will demonstrate that if you know the dew point temperature you can quickly figure out the mixing ratio and vice versa.
| Tair = 90 F |
r = ? |
| RH = ? |
Td = 50 F |
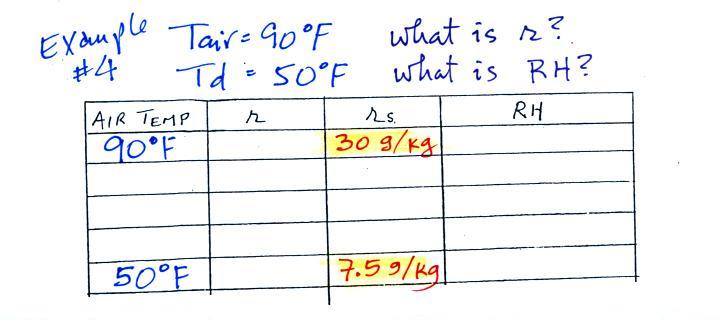
We enter the two temperatures given on a chart and look up the saturation mixing ratio for each.
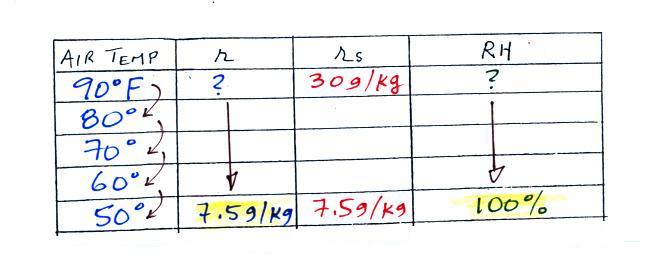
We ignore the fact that we don't know the mixing ratio. We do know that if we cool the 90 F air to 50 F the RH will become 100%. So on the 50 F row, we can set the mixing ratio equal to the value of the saturation mixing ratio at 50 F, 7.5 g/kg. The two have to be equal in order for the RH to be 100%.
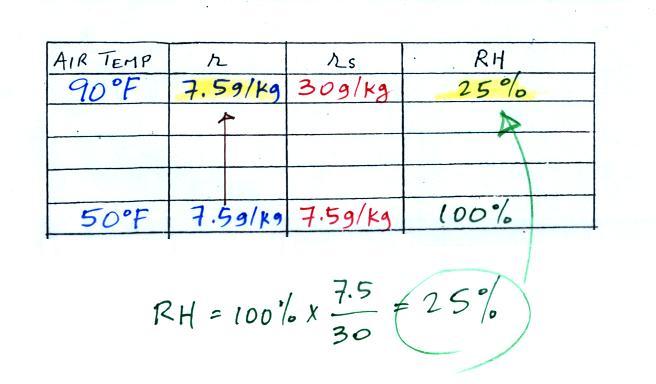
Remember back to the three earlier examples. When we cooled air to the the dew point, the mixing ratio didn't change. So the mixing ratio must have been 7.5 all along. Once we know the mixing ratio in the 90 F air it is a simple matter to calculate the relative humidity, 25%.
Drying moist air
The figure below has been squeezed into the left side of page 87 in the ClassNotes. It explains how you can dry moist air. This is something that Mother Nature does all the time.
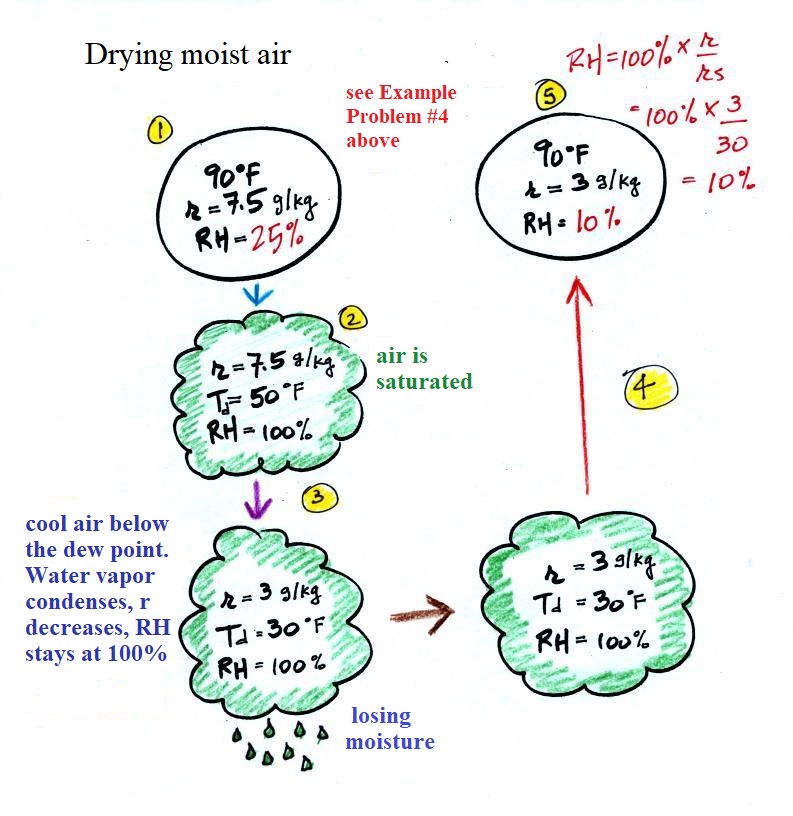
At Point 1 we start with some 90 F air with a relative humidity of 25%, fairly dry air. These are the same numbers that we had in Example Problem #4. We imagine cooling this air to the dew point temperature, 50 F. While doing that the mixing ratio, r, would stay constant. Relative humidity would increase and eventually reach 100%. A cloud would form (Pt. 2 in the figure above).
Then we continue to cool the air below the dew point, to 30 F. Air that is cooled below the dew point finds itself with more water vapor than it can contain. The excess moisture must condense (we will assume it falls out of the air as rain or snow). Mixing ratio will decrease, the relative humidity will remain 100%. When air reaches 30 F it contains 3 g/kg, 40% of the moisture that it originally did (7.5 g/kg).
The air is being warmed back up to 90 F along Path 4. As it warms the mixing ratio remains constant. Cooling moist air raises the RH. Warming moist air, as is being done here, lowers the RH. Once back at the starting temperature, Point 5, the air now has a RH of only 10%.
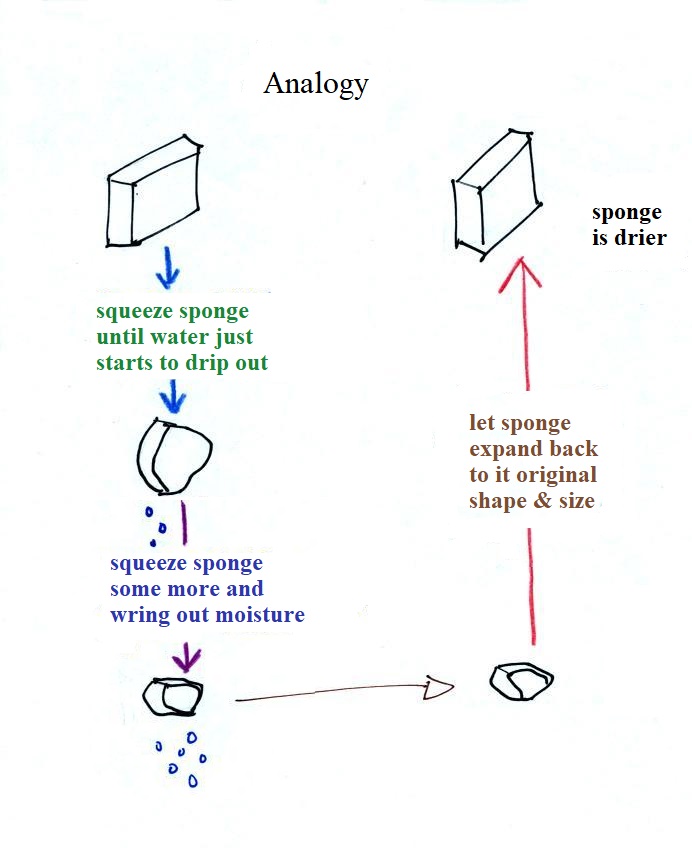
Drying moist air in this way is basically like wringing moisture from a wet sponge.
Dry air indoors in the winter
The air indoors in the winter is often quite dry (low values of the mixing ratio and relative humidity).
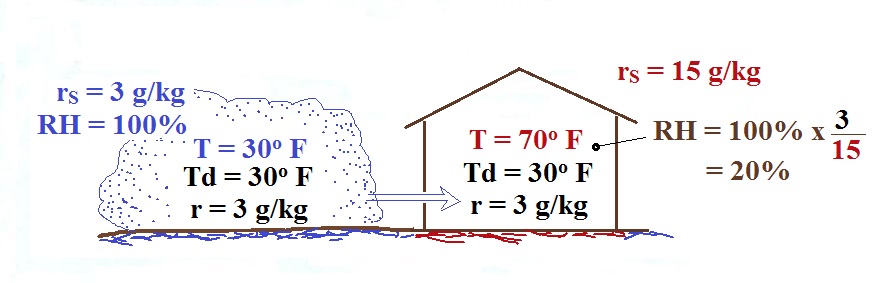
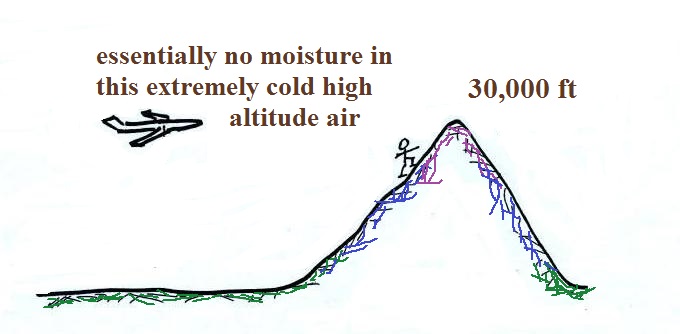
The air in an airplane comes from
outside the plane. The air outside the plane can
be very cold (-60 F perhaps) and contains very little
water vapor (even if the -60 F air is saturated it would
contain essentially no water vapor). When brought
inside and warmed to a comfortable temperature, the RH
of the air in the plane would be essentially 0%.
The RH doesn't get this low because the airplane adds
moisture to the air to make to make the cabin
environment tolerable. Still the RH of the air
inside the plane is pretty low and passengers often
complain of dehydration
on long airplane flights. This
may increase the risk of catching a cold (ref)
The rain-shadow effect
Next a much more important example of drying moist air (see page 88 in the ClassNotes).
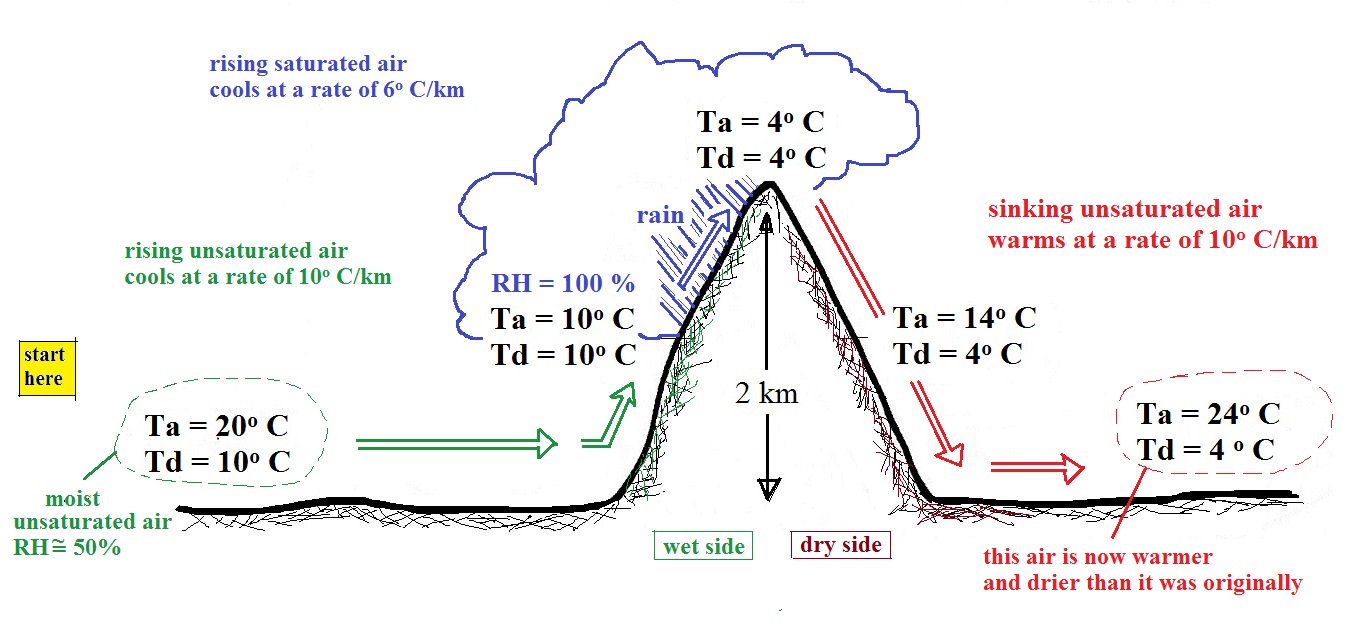
The air becomes saturated mid way up the mountain (the air temperature and the dew point are both 10 C). Would you be able to tell if you were outdoors looking at the mountain? Yes, you would see a cloud appear.
Now that the RH = 100%, the saturated air cools at a slower rate than unsaturated air (condensation of water vapor releases latent heat energy inside the rising volume of air, this warming partly offsets the cooling caused by expansion). We'll use a value of 6 C/km (an average value). The air cools from 10 C to 4 C in next kilometer up to the top of the mountain. Because the air is being cooled below its dew point, some of the water vapor will condense and fall to the ground as rain. Moisture is being removed from the air and the value of the mixing ratio (and the dew point temperature) decreases.
The air starts back down the right side of the mountain. Sinking air is compressed and warms. As soon as the air starts to sink and warm, the relative humidity drops below 100% and the cloud disappears. The sinking unsaturated air will warm at the 10 C/km rate.
After passing up and over the mountain, the air ends up warmer (24 C vs 20 C) and drier (Td = 4 C vs Td = 10 C) than when it started out. The downwind side of the mountain is referred to as a "rain shadow" because rain is less likely there than on the upwind side of the mountain. Rain is less likely because the air is sinking and because the air on the downwind side is drier than it was on the upslope side.
 |
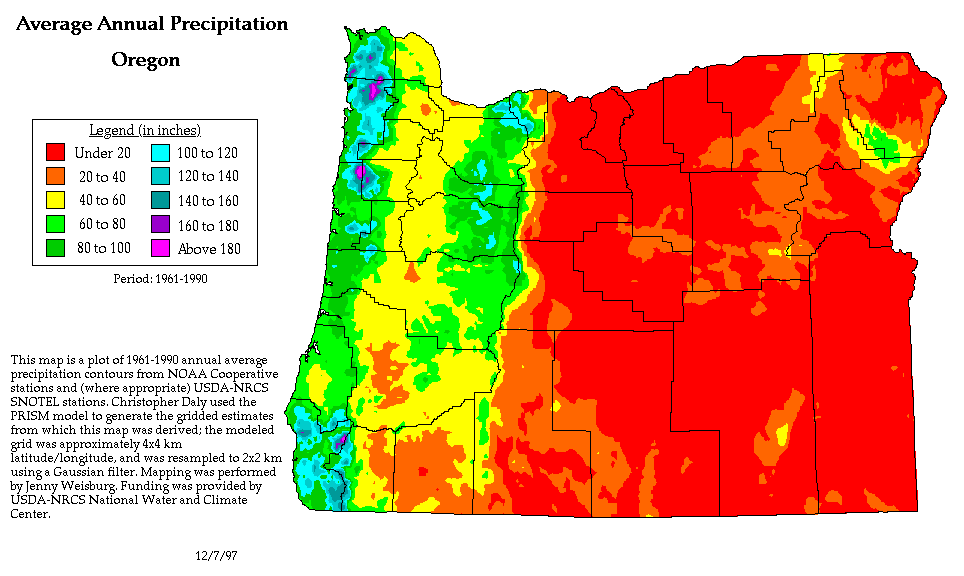 |
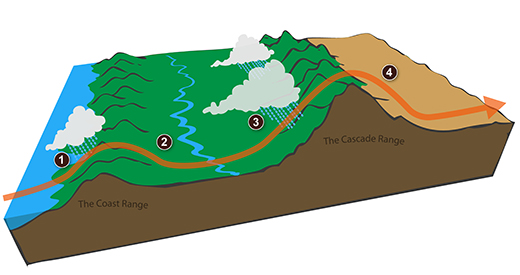
We can see the effects of a rain shadow illustrated well in the state of Oregon. The figure above at left shows the topography (here's the source of that map). Winds generally blow from west to east across the state.
Coming off the Pacific Ocean the winds first encounter a coastal range of mountains. On the precipitation map above at right (source) you see a lot of greens and blue on the western sides of the coastal range. These colors indicate yearly rainfall totals that range from about 50 to more than 180 inches of rain per year. Temperate rainforests are found in some of these coastal locations. The line separating the green and yellow on the left side of the precipitation map is the summit, the ridgeline, of the coastal mountain range.
That's the Willamette River valley, I think, in between the coastal range and the Cascades. This valley is somewhat drier than the coast because air moving off the Pacific has lost some of its moisture moving over the coastal range.
What moisture does remain in the air is removed as the winds move up and over the taller Cascades. The boundary between yellow/green and the red is the ridgeline of the Cascade Mountains. Yearly rainfall is generally less than 20 inches per year on the eastern side, the rain shadow side, of the Cascades. That's not too much more than Tucson which averages about 12 inches of rain a year.
 |
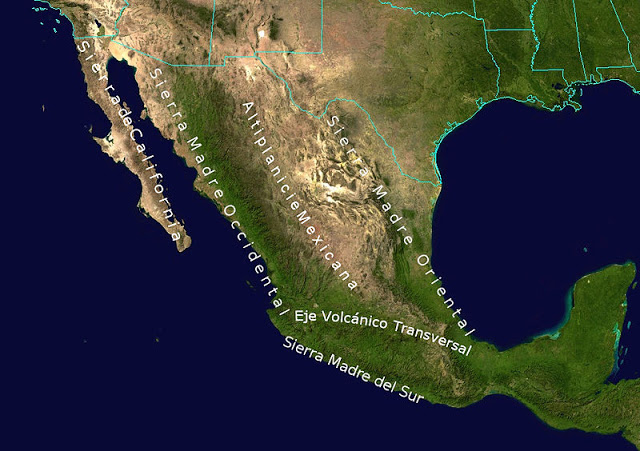 |
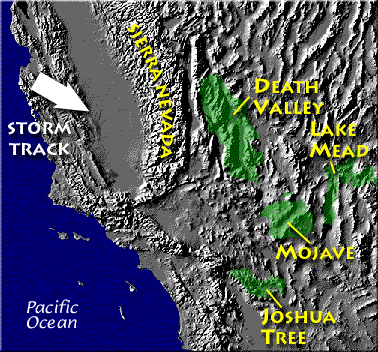
Death valley is found on the downwind side of the Sierra Nevada mountains (source of left image). The Chihuahuan desert and the Sonoran desert are found downwind of the Sierra Madre mountains in Mexico (source of the right image). Mexico might be a little harder to figure out because moist air can move into the interior of the country from the east and west at different times of the year. It does appear that the eastern slopes of the two mountain ranges are the wettest so much of that precipitation must come from moist air moving in from the east (rising air motions on the eastern mountain slopes).
Most of the year, the air that arrives in Arizona comes from the west, from the Pacific Ocean (this changes in the summer). It usually isn't very moist by the time it reaches Arizona because it has traveled up and over the Sierra Nevada mountains in California and the Sierra Madre mountains further south in Mexico. The air loses much of its moisture on the western slopes of those mountains. Beginning in early July in southern Arizona we start to get air coming from the south or southeast. This air can be much moister and leads to development of our summer thunderstorms.
Just as some of the world's driest regions are found on the downwind side (the rain shadow side) of mountain ranges, some of the wettest locations on earth are on the upwind sides of mountains. There seems to be some debate whether Mt. Wai'ale'ale in Hawaii or Cherrapunji India gets the most rain per year. Both get between 450 and 500 inches of rain per year.