Now back to ozone
Here's a good complete discussion of the different UV
Index levels from the US Environmental Protection Agency (https://www.epa.gov/sunsafety/uv-index-scale-0)
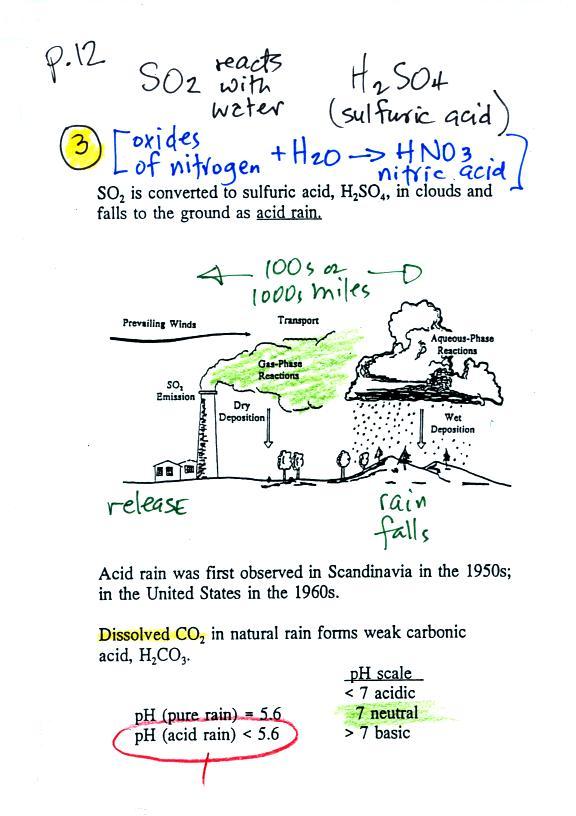
Ozone in the troposphere (surface level ozone in the
figure above) is bad, it is toxic and a pollutant.
Tropospheric ozone is also a key component of
photochemical smog (also known as Los Angeles-type smog)
We'll be making some photochemical smog in a class
demonstration. To do this we'll first need some
ozone; we'll make use of the simple stratospheric recipe
for making what we need instead of the more complex
tropospheric process (the 4-step process in the figure
below). You'll find more details a little further
down in the notes.
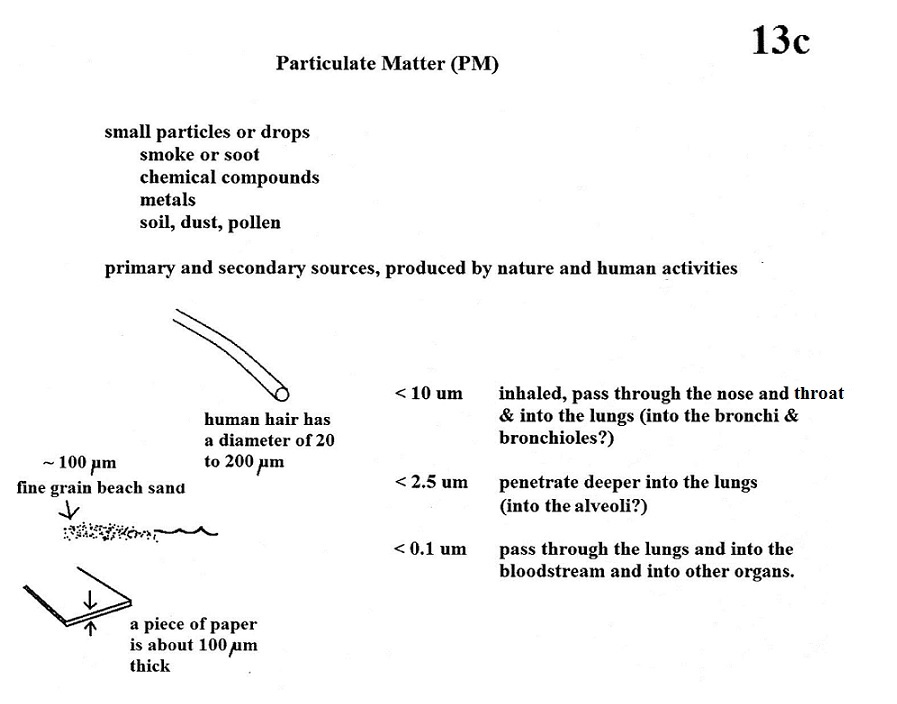
A more complex series of reactions is responsible for the
production of tropospheric ozone. The
production of tropospheric ozone begins with nitric oxide
(NO). NO is produced when nitrogen and oxygen in air
are heated (in an automobile engine for example) and
react.
The NO can then react with oxygen in the air to make
nitrogen dioxide, the poisonous brown-colored
gas that I used to make in class.
Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2 react in a 4th step
to make ozone (O3)
just like happens in the stratosphere. Because ozone
does not come directly from an automobile tailpipe or
factory chimney, but only shows up after a series of
reactions in the air, it is a secondary
pollutant. Nitric oxide (NO) would be the
primary pollutant in this example.
NO is produced early in the day (during the morning
rush hour). The concentration of NO2 peaks somewhat
later. Because sunlight is needed in step #3 and
because sunlight is usually most intense at noon, the
highest ozone concentrations are usually found in the
afternoon. Ozone concentrations are also usually
higher in the summer when the sunlight is more intense
than at other times of year.
The American Lung Association's 2018 State of the Air
report mentions that 11 of the 25 cities with the highest
tropospheric ozone concentrations are found in California
(Los Angeles is at the top of the list). Two cities
are found in both Texas and Colorado, one each in Arizona
(Phoenix), Utah and Nevada. Here's a link to the full
report (167 pages long). Here's a shorter
report with the lists of most
polluted cities. Here a list of the cities in
the United States with the cleanest
air.
Earlier this month Tucson exceeded the EPA NAAQS for
ozone for the first time. Phoenix had already exceeded the
NAAQS 39 times this year. Here's a link to the entire
article.

The violation in Tucson, which
could impact the availability of federal
transportation funds, is partly because the allowed
ozone concentration is lower than it used to be (80
parts per billion (ppb) averaged over an 8 hour period
to 75 ppb in 2008 to 70 ppb in 2015).
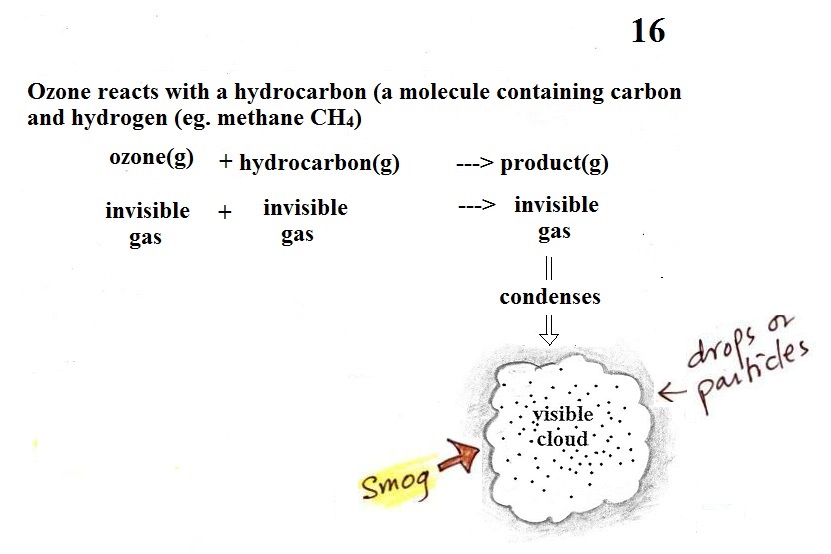
Once ozone is formed, the ozone can
react with a hydrocarbon of some kind to make a product
gas. The ozone, hydrocarbon, and product gas are all
invisible, but the product gas sometimes condenses to make
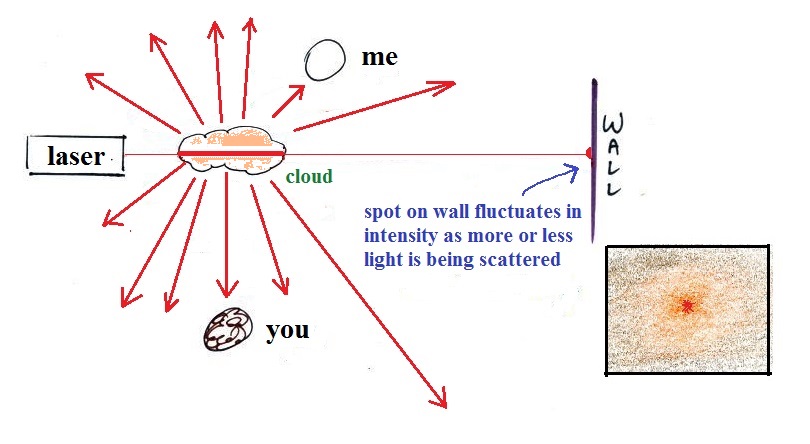
a visible smog cloud or haze. The cloud is composed
of very small droplets or solid particles. They're
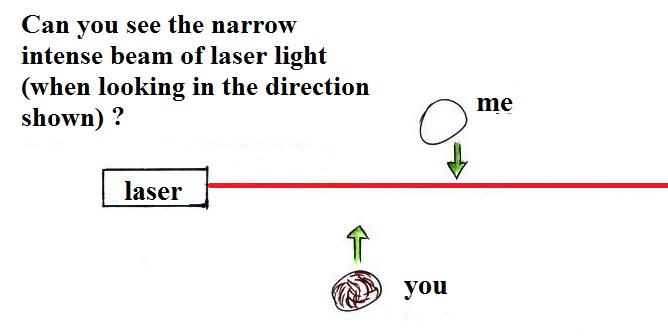
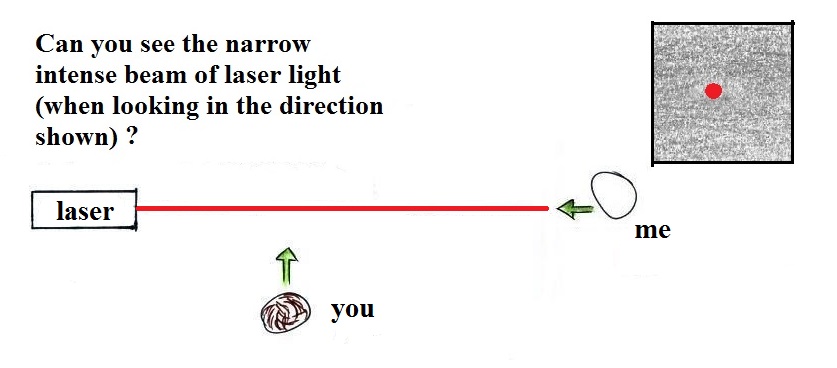
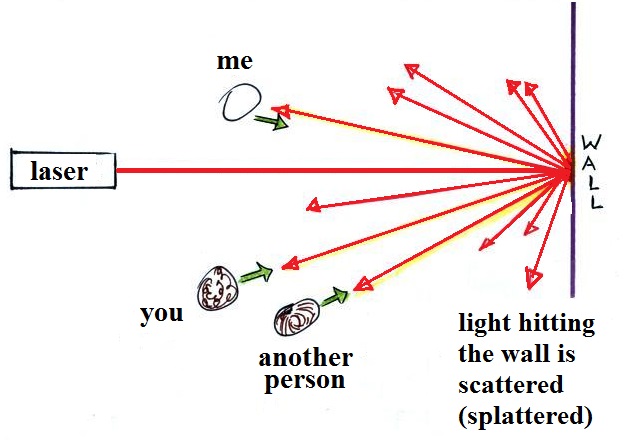
too small to be seen but they are able to scatter light -
that's why you can see the cloud.
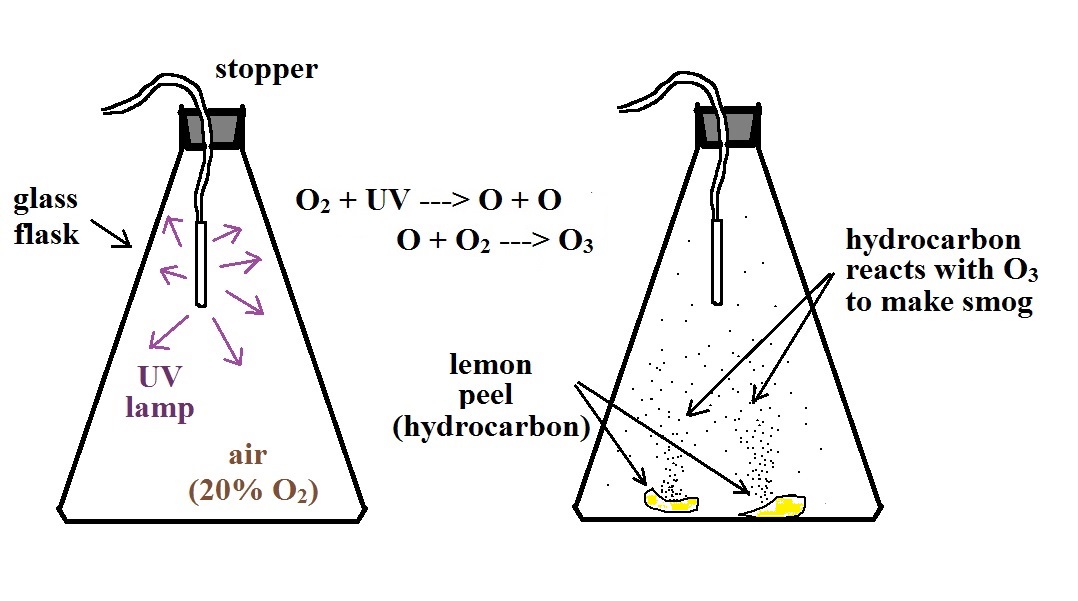
We started by putting a small "mercury vapor" lamp
inside a flash. The bulb produces a lot of
ultraviolet light (the bulb produced a dim bluish light
that we could see, but the UV light is invisible so we had
no way of really telling how bright the bulb was).
The UV light and oxygen in the air produced a lot of ozone
(you could have easily smelled it if you had taken the
cover off the flask).
After a few minutes we turned off the
lamp and put a few pieces of lemon peel into the
flash. Part of the smell of lemon is limonene, a
hydrocarbon. The limonene gas reacted with the ozone
to produce a product gas of some kind. The product
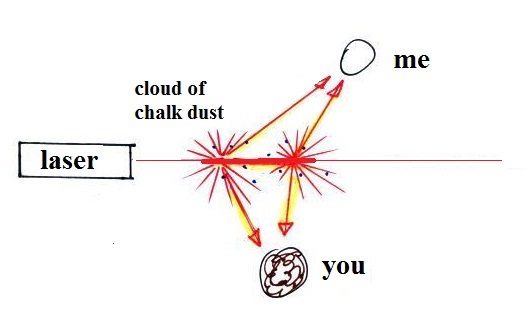
gas condensed, producing a visible smog cloud We
shined the laser beam through the smog cloud to reinforce
the idea that we are seeing the cloud because the drops or
particles scatter light.