The next section of material concerns water in the atmosphere. We will begin by describing the physics behind the important processes of evaporation and condensation. The concepts of relative humidity and dew point temperature will be explained. We will then show how the combination of air temperature, humidity, and winds relate to human comfort or discomfort and what is meant by windchill temperature and heat index. Next the process of cloud formation will be described. This will lead us to severe weather development since the heat released as clouds form provides much of the the energy for thunderstorms, tornadoes, and hurricanes.
Water exists in all three phase states (gas, liquid, and solid) at the temperatures commonly found on the Earth and in its atmosphere. The concentration of the invisible gas water vapor varies greatly from place to place, and from time to time. In warm tropical locations close to the surface, water vapor can be up to 4% of the atmospheric gases. In cold polar regions, there is just a trace amount. Much of the water on Earth exists in the liquid phase and most of that is contained in vast oceans. Liquid water is also found in lakes, in soils, and cloud droplets. Ice, the solid phase of water, is found in glaciers, sea ice, ice on land, and as ice crystals in cloud particles and snow.
Water vapor is extremely important in the atmosphere for many reasons. A few of those reasons include:
Remember from the kinetic model that the molecules that compose a substance are in constant motion due to the internal energy possessed by the molecules and that there are three different "states" or "phases" of matter, which are solid, liquid, and gas. The physical state (or phase) is determined by the relative strength of the molecular internal energy and the energy of attraction between molecules. At low temperature (and low internal energy per molecule), the energy of attraction is stronger than the internal energy, and the molecules bond together to form a solid. The molecules in a solid can vibrate, but they stay in a fixed position. At higher temperature (and internal energy per molecule), the internal energy is similar in strength to the energy of attraction, which changes the bonds between molecules so that the substance can move more freely as a liquid. In a liquid, the molecules can vibrate, rotate and move around, but they remain in close contact through chemical bonds. Liquids therefore have fixed volumes, but not fixed shapes. At high temperature (and high internal energy per molecule), the internal energy is stronger than the energy of attraction, the bonds between molecules break, and the substance is in the gas state. In a gas the individual molecules are not bonded together, so they can move about freely in space in addition to rotating and vibrating. Gases do not have fixed volumes or shapes and thus take on the shape and volume of the container holding them.
Water occurs in all three phases on Earth, as a solid (ice), a liquid, and a gas (water vapor). Water also readily changes phase in response to changes in internal energy. When water molecules have low internal energy, they bind together in a crystalline ice structure. Adding energy (or heat) to ice, raises its internal energy, which "loosens" or rearranges the molecular bonding, so that the molecules move more freely as a liquid. Adding more energy (or heat) to liquid water, raises its internal energy to the point the molecular bonds break and the substance becomes a gas, known as water vapor. Water vapor is simply water in the gas phase, i.e., individual molecules of H2O become part of the mixture of gases in the atmosphere.
The diagram below shows the energy that must be absorbed or released for the substance water to change phase. The gas form water vapor has the highest internal energy per gram of water, followed by liquid water, and then ice. Any phase change from a lower to a higher internal energy state requires the addition of energy (heat), while any phase change from a higher to a lower internal energy state involves a release of energy (heat). The energy that must be added to or removed from water as it changes phases is called latent heat. The latent heat absorbed or released by water is necessary because the water molecules must change their chemical bonding patterns and internal energy to change phase. Thus, latent heat refers only to the energy that must be added to or removed from water to cause phase changes. Latent heat does not cause the temperature of water to change, only the phase. Energy which is used to change the measured temperature of a substance, but not its phase, is called sensible heat. For example, to raise the temperature of liquid water from 20°C to 30°C requires the addition of sensible heat and to lower the temperature of liquid water from 30°C to 20°C requires the removal of sensible heat.
| Deposition 680 cal/g Energy Released to environment (heating) |
||||
 |
||||
 |
Freezing 80 cal/g  Melting |
 |
Condensation 590 cal/g  Evaporation |
 |
 |
||||
| Energy taken from Environment (cooling) Sublimation 680 cal/g |
||||
The calorie (cal) is a physical unit for measuring energy. It is defined as the amount of energy required to raise the temperature or one gram of liquid water by 1 degree Celsius. The diagram above shows the amount of latent energy required for phase changes of water per gram of water. Phase changes from lower to higher energy phases of water, melting, evaporation, and sublimation, require energy to be added to the water. Phase changes from higher energy to lower energy phases of water, condensation, freezing, and deposition, requires that energy be removed from the water. It is very important to realize the relative amounts of latent heat involved in the various phase changes. The latent heat assoicated with the phase change between solid (ice) and liquid water is 80 calories per gram of water. The latent heat associated with the phase change between water vapor (gas form of water) and liquid water is 590 calories per gram of water. The tremendous amount of energy involved in evaporation and condensation of water is very important in understanding the operation of weather and climate on Earth.
During the processes of melting, evaporation, and sublimation, water absorbs energy. The energy absorbed causes the water molecules to change their bonding pattern and transform to a higher energy state. In the Earth system, this energy must be supplied by the surrounding environment. Thus, these phase changes result in cooling of the surrounding environment. In other words water is absorbing energy from its surrounding environment (to undergo these phase changes). Since the surrounding environment is losing energy, it cools down.
During the processes of condensation, freezing, and deposition, water releases energy. The energy released allows the water molecules to change their bonding pattern and transform to a lower energy state. In the Earth system, this energy must be absorbed by the surrounding environment. Thus, these phase changes result in warming of the surrounding environment. In other words water is releasing energy to its surrounding environment (to undergo these phase changes). Since the surrounding environment is absorbing or gaining energy, it warms up.
In the atmosphere, phase changes between liquid and gas are very important because of the large amount of latent heat involved. Latent heat released by water vapor during condensation in cloud formation is crucial for climate as it is part of the process that moves energy from the ground surface up into the atmosphere. The latent heat released during condensation is also the main energy source for the formation of thunderstorms and hurricanes. Make sure that you understand that evaporation of water results in cooling of the surrounding environment and that condensation of water results in warming the surrounding environment. The last several points are made again in this WORD document on phase changes of water in the atmosphere. I suggest that you read that document.
This is so important that it is worth repeating again! Please do not leave this page and miss these important points! Water must absorb energy to move from a lower to higher energy phase, i.e., from solid (ice) to liquid during melting and from liquid to gas (water vapor) during evaporation. In each case, the energy absorbed by water is used to change its internal molecular bonding structure. The water must absorb this energy from outside, which often results in a cooling of the surrounding environment since it must supply the energy to the water.
Water must release energy to move from a higher to lower energy phase, i.e., from gas (water) vapor to liquid during condensation and from liquid to solid (ice) during freezing. In each case, the energy released by the water results in a change in the internal molecular bonding structure. This released energy is taken up or absorbed by the surrounding environment, which typically results in a warming of the surrounding environment since it has to absorb the energy.
The water cycle refers the continuous movement of water on, above, and below the surface of the Earth. Water can change phase among liquid, gas (water vapor), and solid (ice) at various places in the cycle. The importance of the water cycle for life on land cannot be overstated ... without the water cycle, most of the terrestrial life on Earth could not exist. You should be familar with the basics of the water cycle and the use of the terms evaporation, condensation, precipitation, and evapotranspiration as described in the next paragraph.
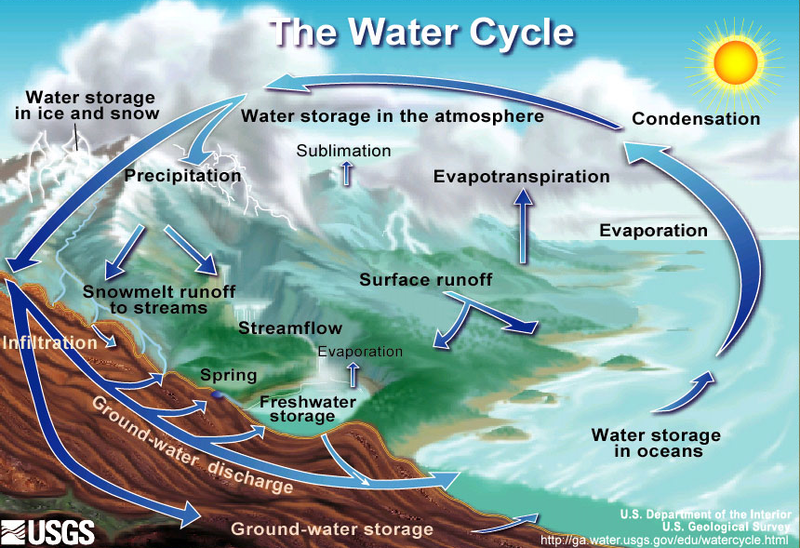
|
Energy from the sun drives the water cycle. (Remember energy must be added to liquid water (absorbed by liquid water) to cause it to evaporate). Liquid water on or slightly below the Earth's surface evaporates into the air, becoming the gas water vapor. Close to 90% of the water vapor in the atmosphere evaporated from the oceans and to a much smaller degree from lakes. Water is also evaporated from the land surface (out of soils) or transpired by plants. These processes on land are often lumped together and called evapotranspiration. Thus, the dominant process near the Earth's surface is evaporation (liquid to gas), acts to remove energy from the Earth's surface. This energy does not just disappear, though. The energy is now contained (or stored) in the water vapor molecules, which have more internal energy than liquid water molecules. Rising air currents carry water vapor up into the atmosphere, which cools the air, causing the water vapor to condense into tiny droplets of liquid water (or tiny ice crystals), forming clouds. This process, condensation (gas to liquid), releases energy up in the atmosphere where clouds form. In other words, now the energy from the surface that was stored in the water vapor molecules is now released during condensation when the water molecules move from a higher state of internal energy (gas) to a lower state of internal energy (liquid). In fact the water cycle transports energy ... removing it from the surface via evaporation and delivering it in the middle and upper troposphere where clouds form. When cloud droplets grow large enough they fall to the surface as precipitation. Much of the water on the surface of the Earth collects in the oceans, but it can also be stored as groundwater or ice. Eventually it evaporates again to again move through the water cycle.