Often, the Earth's atmosphere is divided into several different layers that are defined according to the typical change in air temperature. (See Image Below).
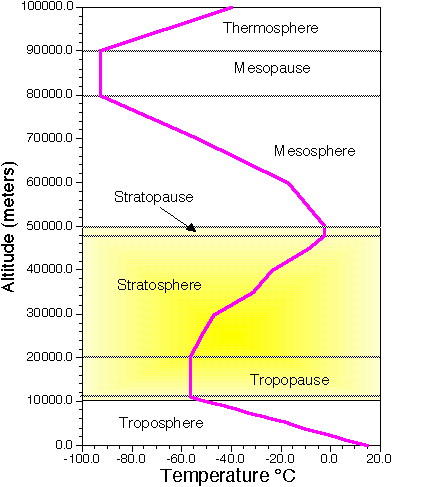 |
| Atmospheric layers defined by structure of air temperature. |
Air density can be defined as the number of air molecules per unit volume (number density). Near sea level there are about 2.7x1019 molecules per cm3(cubic centimeter) or 4.4x1020 molecules per inch3(cubic inch). Air molecules are held near the earth by gravity. In other words, air has weight. Weigh an empty bag, then fill it with air, it now weighs more. In addition gases, like air, are easily compressed, i.e., squeeze a gas together and its number density increases. In other words, we say gases are compressible because they can easily be squeezed into a smaller volume. Solids and liquids on the other hand are not easily compressed.
![[pgf]](density_column.png)
|
| Density of dots represents the decrease in air number density at higher altitudes |
The weight of all of the air above a given point in the atmosphere squeezes air molecules closer together, which causes their numbers in a given volume to increase (increase in number density). The more air above a level (and hence the more weight of air above a level), the greater the squeezing effect (or compression).
Since air density is the number of air molecules in a given space (volume), air density is typically greatest at the surface or sea level (where it is squeezed by the weight of the entire atmosphere above) and decreases as we move up in the atmosphere because the weight of air above becomes less and hence there is less of a squeezing effect (See Figure Z). The decrease in air number density with increasing elevation is also depicted in the figure on the right.
Atmospheric air pressure results from the Earth's gravitational pull on the overlying air. Without gravity holding the atmosphere just above the ground surface, air molecules would spread out, and the gas pressure would be close to zero.
The weight of the atmosphere acts as a force upon the underlying surface of the Earth. The amount of force excerted over an area of surface is called atmospheric pressure or air pressure. Near sea level, the average air pressure is about 14.7 pounds per square inch. In this class we will use the unit millibars(mb) to specify air pressure. At sea level the average air pressure is 1013 mb. Another way to think of this is that on average the total weight of all the air above sea levels weighs enough to cause 1013 mb of air pressure. Please keep in mind that 1013 mb is just the average air pressure at an altitude of sea level. It is very important to realize that the air pressure is not constant at a given altitude, e.g., sea level pressure commonly ranges from 980 mb to 1045 mb, since variations in air pressure along a horizontal surface cause horizontal winds to blow.
Since the air (a gas) is a fluid, the pressure force acts in all directions, not just downward. The pressure force pushing downward due to the weight of the air is the same as the pressure force acting sideways and even upward. If you are having trouble understanding this, make an analogy with another fluid liquid water. Consider a deep swimming pool full of water. The water pressure anywhere in the pool depends on the weight of the water above (that is the deeper you dive downward in the pool, the stronger the water pressure.) The pressure force is not just downward though, it pushes in on your body from all directions. The average air pressure at sea level (1013 mb or sometimes called one atmosphere of pressure) is caused by the weight of all the air above sea level. In the same way water pressure is caused by the weight of water above you. At a depth of 32 feet (9.75 meters) below a water surface, the water pressure is about 1013 mb, which is known as one atmosphere of air pressure. Thus, the entire column of air from sea level to outer space weighs as much as a 32 foot column of water. Of course diving deeper than 32 feet downward into water means you will encouter an increasing water pressure (enough to crush you if you go too deep). And yes the total pressure under water would include both the overlying air pressure (about 1013 mb at sea level) plus the added water pressure.
 |
| Typical change in air pressure with altitude. Note how rapidly air pressure falls with increasing altitude. |
In the atmosphere, the air pressure at any point is caused by the weight per area of the air above that point. As we climb in elevation, fewer air molecules are above us (less weight of air above us); hence, atmospheric pressure always decreases as you move upward in the atmosphere (See Figure B). Another way to look at it is that the air pressure at any point in the atmosphere is exactly enough to support the weight of the column of air above it. A balance exists between the gravitational force pushing air downward and the upward directed pressure force. This balance is called hydrostatic balance (see figure).
Earlier we made an analogy between diving down in water and moving downward in the atmosphere. In both cases, the fluid presure increases as you move down because there is more and more weight of fluid above you. A big difference between water and air, though, is that air is compressible and water is not. This affects the rate of pressure changes as one moves up or down in the fluid as shown in Figure C, which compares the rate of vertical pressure changes between water and air.
Because air is compressed by its own weight, much of the mass of the atmosphere is squeezed into the troposphere where the air is most dense (higher number density), while only a small portion of the mass of the atmosphere remains above the stratosphere where air is less dense (lower number density). Since air pressure is directly related to the weight of air above a given point, a ratio of air pressure is equivalent to a ratio of weight.
| Measured air pressure at your location | ||
| Fraction of the atmosphere above you by weight | = | |
If you do not know the sea level air pressure, you can 1000 mb as a good approximation. Thus, at a location where the air pressure is 500 mb, roughly half the weight of the atmosphere is above you and the other half is below you. A typical 500 mb height is about 5500 meters or 5.5 km above sea level. Thus, half the weight of the atmosphere is compressed into the vertical column from sea level up to about 5.5 km above the surface while the other half is spread out from 5.5 km upward to the top of the atmosphere, somewhere around 500 km above sea level. This happens because the number density is greatest just above the ground surface and decreases as you move upward. Note in the figure above how rapidly air pressure drops as you move up above the surface where number density is largest, but that the rate of pressure drop slows down as you move to higher altitudes where the number density is much smaller. This is characteristic of an exponential decrease in air pressure with increasing height. On average in Earth's atmosphere, the air pressure approximately drops in half for every 5.5 km increase in altitude. The air pressure is roughly 1000 mb at sea level, 500 mb at 5.5 km above sea level, 250 mb at 11 km above sea level, 125 mb at 16.5 km above sea level, and so forth. The figure above also shows that on average the top of the troposphere is located at a pressure of about 250 mb. Using the equation above, this means that 25% of the mass of the atmosphere is above the troposphere and 75% of the mass of the atmosphere is contained in the troposphere as stated in the temperature section above.