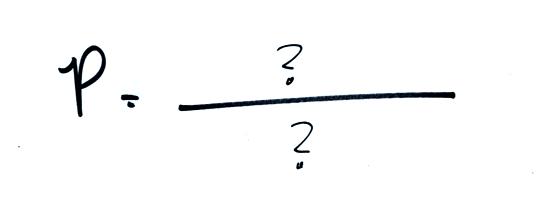Wednesday Jan. 30, 2013
click here to download today's notes
in a more printer friendly format
Robert Plant and Alison Krauss "When the
Levee Breaks" before class. We didn't have time
for "Sister Rosetta
Goes Before Us" but I've included a link anyways.
The 1S1P reports on Stratospheric Ozone are due Friday this
week. I should have the 3rd topic ready by then also.
The Expt. #1 reports are due next Monday. Try to return your
materials this week so that you can pick up the Supplementary
Information handout before you start to write your report. I
am planning to hand out the Expt. #2 materials sometime next week
(probably Friday).
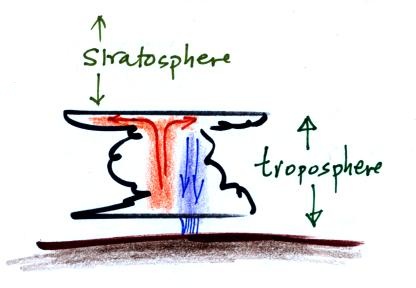
Thunderstorms form when the atmosphere is unstable (lots of up
and down air motions). They grow upward until they reach the
bottom of the (very stable) stratosphere and then spread out
sideways to form an anvil cloud. Photo of a thunderstorm
anvil as seen from the Space Station (source)
A fairly ambitious class coming up on Friday when our objective
will be to understand why warm air rises and cold air sinks.
We'll get some of the preliminary and introductory material out of
the way today before the Practice Quiz.

Hot air balloons rise (they also sink), so does
the relatively warm air in a thunderstorm updraft (it's
warmer than the air around it). Conversely cold
air sinks. The surface winds caused by a thunderstorm
downdraft (as shown above) can reach speeds of 100 MPH
(stronger than most tornadoes) and are a serious weather
hazard.
A full understanding of these rising and
sinking motions is a 3-step process (the following is from
the bottom part of p. 49 in the photocopied
ClassNotes).
1.
We will first learn about the ideal gas law. That is an
equation that tells you which properties of the air inside a
balloon work to determine the air's pressure.
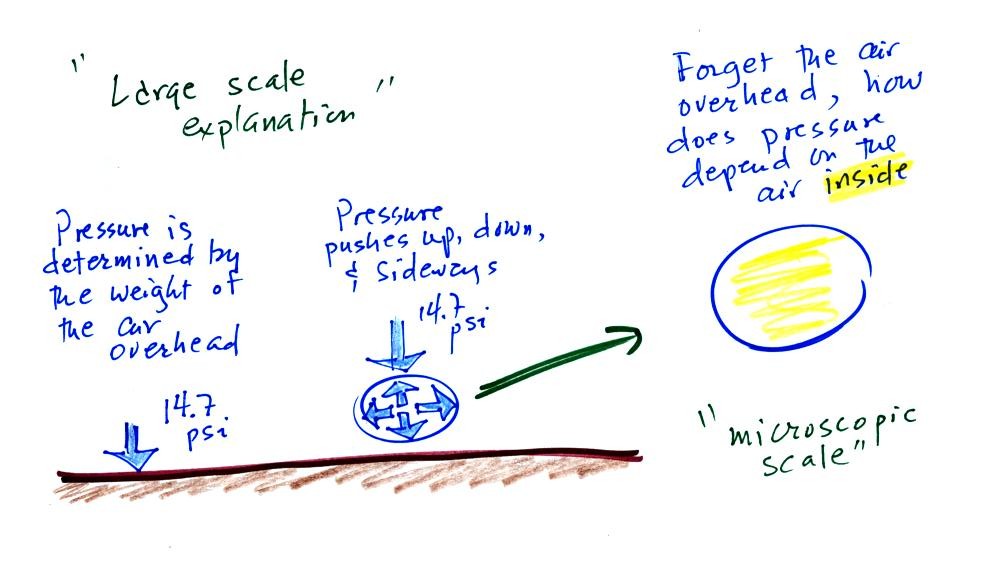
We first learned to think about pressure as being
determined by the weight of the air overhead. Air
pressure pushes down against the ground at sea level with
14.7 pounds of force per square inch. That's a
perfectly good concept.
We then went a bit further and tried to imagine the weight
of the atmosphere pushing down on a balloon sitting on the
ground. If you actually do push on a balloon you
realize that the air in the balloon pushes back with the
same force. Air everywhere in the atmosphere pushes
upwards, downwards, and sideways.
Next we will ignore the
rest of the atmosphere and concentrate on just the air
inside the balloon. We'll end up with an
equation. Pressure (P) will be on the left hand side
of the equation. The right hand side of the equation will
show how various properties of the air in the balloon work
to determine the air pressure.
Students working on Experiment #1
will need to understand the ideal gas law to be able to
fully explain why/how their experiment works.
2.
Then we will look at Charles' Law, a special situation involving
the ideal gas law (air temperature volume, and density change
together in a way that keeps the pressure inside a balloon
constant). This is important because air in the atmosphere
obeys Charles' Law.
3.
Finally we'll learn about the vertical forces that act on air
(an upward and a downward force).
You should already know what two forces are
involved (gravity and the upward pointing pressure
difference force).
The remainder of today's class was taken up by the Practice Quiz. I would
recommend that you have a look at the Practice Quiz if you
weren't in class just so that you can become familiar with the
quiz format.
Answers to the questions on the Practice Quiz will appear online
sometime before class on Friday.