Tuesday Sept. 3, 2019
Junius Meyvant "Be A Man"
(3:05), "Mighty
Backbone" (3:36), "Gold Laces"
(3:51), "Color
Decay" (4:28); Ylja "Ut"
(3:33)
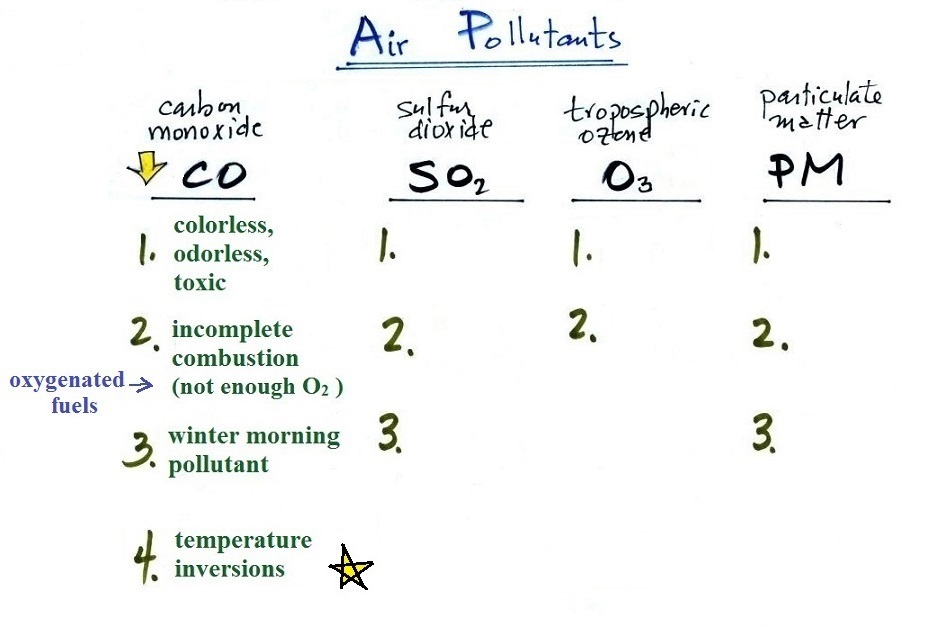
We'll use page
11, page12, page14a, page 15, and page 16 from the ClassNotes
today. I'll probably also bring page 13c just in case we
finish the material below with a little extra time left in class.
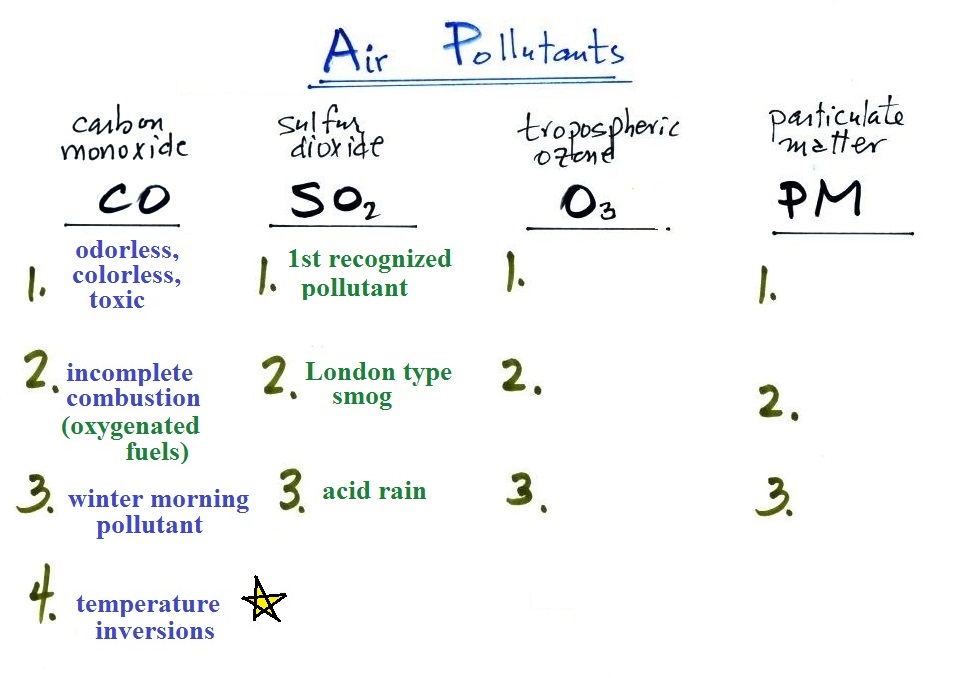
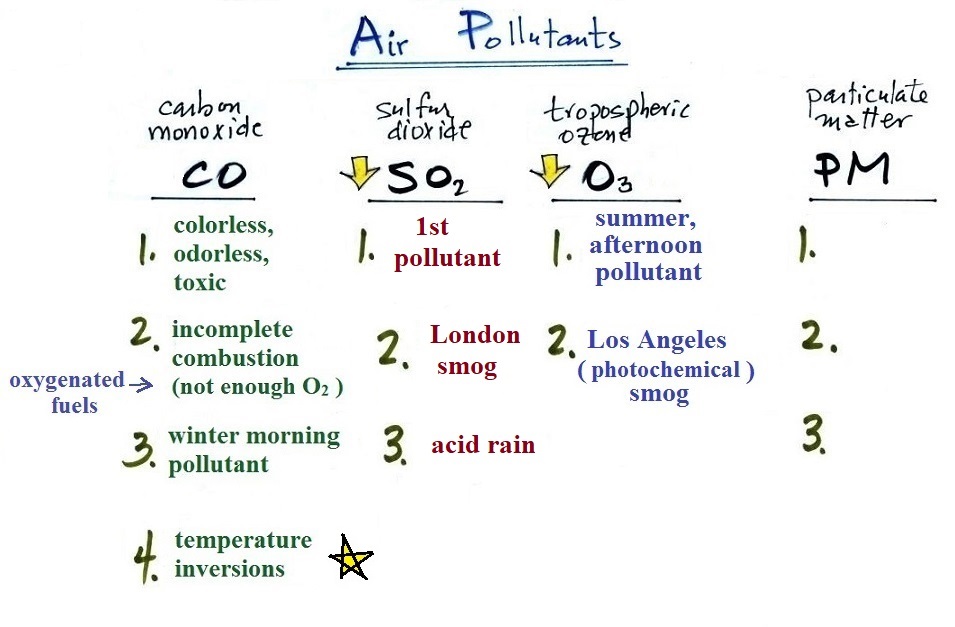
A quick summary of the key points concerning carbon
monoxide. We'll cover sulfur dioxide and tropospheric ozone
today.
The yellow star next to temperature inversions means it's a
pretty important concept and well worth trying to remember.
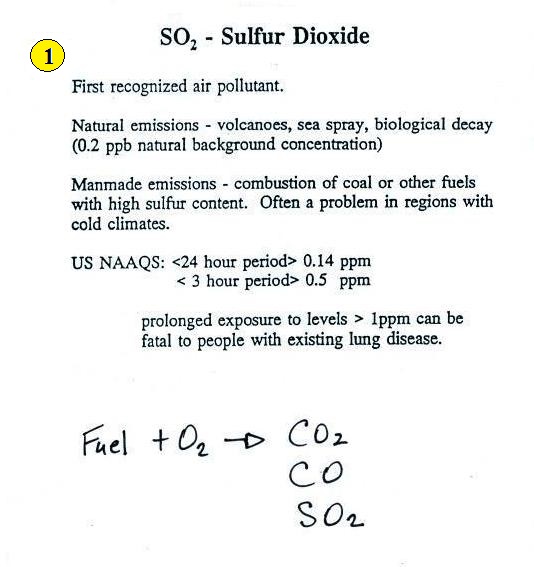
Sulfur dioxide (SO2
)
We'll turn now to another of the air
pollutants, sulfur
dioxide (SO2 ).
See page 11
in the ClassNotes.
Sulfur dioxide is produced by the combustion of sulfur
containing fuels such as coal. Combustion of fuel also
produces carbon dioxide and carbon monoxide. People probably
first became aware of sulfur dioxide because it has an unpleasant
smell (one of the smells in a freshly struck match). Carbon
dioxide and carbon monoxide are odorless. That is most
likely why sulfur dioxide was the first pollutant people became
aware of.
Volcanoes are a natural source of sulfur dioxide.
US NAAQS in the figure above stands for United States National
Ambient Air Quality Standards. Air with a
pollutant concentration that exceeds the NAAQS is considered
unhealthy. This is discussed further in an online Supplementary Reading
section.
London-type smog

The inversion layer in this case lasted for several days and was
produced in a different way than the surface radiation
inversions we heard about when covering carbon monoxide.
Surface radiation inversions usually only last for a few hours.
The term smog, a contraction of smoke + fog, was invented to
describe a mixture of smoke and fog, something that was fairly
common in the winter in London. The 1952 event was an
extreme case. Now we distinguish between "London-type
smog" which contains sulfur dioxide and photochemical or "Los
Angeles-type smog" which contains ozone.
Most of the photographs below come
from articles published in 2002 and 2012, the
50th and 60th anniversaries of the event.
The dramatic drops in visibility are mostly being caused by
fog. Later in the semester we will learn that fog clouds
that form in "dirty" containing certain types of smoke particles
can be thicker than fog that forms in cleaner air.
The caption to this
photo from The Guardian reads
"Arsenal goalkeeper Jack Kelsey peers into the
fog.
The 'smog' was so thick the game was eventually
stopped."
|

The smog in this photo is the thickest I was able to
find. Visibility here is perhaps 10 or 20 feet.
(source
of this image)
|
|
|
|
Smog masks from this
reference
The masks would filter out the smoke but not the
sulfur dioxide gas
|
Even though it is a little off topic, here are
some interesting photographs
of early and mid 20th century London.
The sulfur dioxide didn't
kill people directly. Rather it
would aggravate an existing condition of some
kind. The SO2 probably also
made people susceptible to bacterial infections
such as pneumonia. Here's a link
to a Power Point presentation that
discusses the event and its health effects in more
detail.
The Clean
Air Act of 1956 in England reduced smoke
pollution and emissions of sulfur dioxide.
Air
pollution disasters involving sulfur dioxide have also
occurred in the US. One of the deadliest events was in
1948 in Donora, Pennsylvania.
The reference
material that contained this photographed stated
"This eerie photograph was taken at noon on
Oct. 29, 1948 in Donora, PA as deadly smog enveloped the
town. 20 people were asphyxiated and more than 7,000
became seriously ill during this horrible event."
The photograph below shows some of the mills that were operating
in Donora at the time. Not only where the factories adding
pollutants to the air they were undoubtedly adding hazardous
chemicals to the water in the nearby river.
source
of this photo
The Cuyahoga River that runs through
Cleveland was so polluted that it used to frequently catch
fire (see https://time.com/3921976/cuyahoga-fire/).
It has been 50 years since the last fire and the river has
been cleaned up and restored (see https://www.nytimes.com/2019/06/07/travel/cleveland-cuyahoga-river-pollution.html).
The US passed its own
Clean Air Act in 1963. There have been several
major revisions since then. The EPA began
in late 1970 (following an executive order signed by President
Nixon)
"When
Smoke Ran Like Water" a book about air
pollution is one of the books that you can check
out, read, and report on to replace the
Experiment Report writing requirement in this
class (though I would encourage you to do an
experiment instead). The author, Devra
Davis, lived in Donora Pennsylvania at the time
of the 1948 air pollution episode.
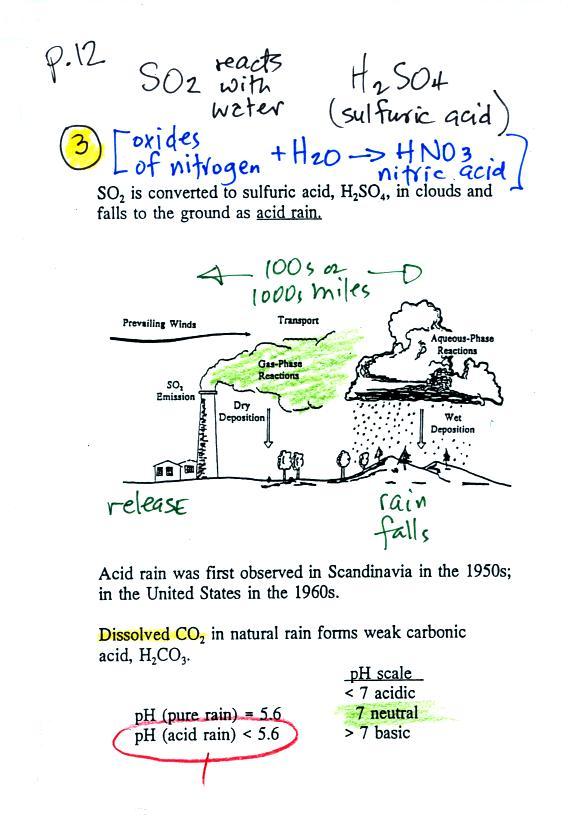
Acid rain
Sulfur
dioxide is one of the pollutants that can react with
water in clouds to form acid rain (some of the
oxides of nitrogen can also react with water to form
nitric acid). The formation and effects of
acid rain are discussed on page 12 in the
photocopied Class Notes.
Acid rain is often a problem in regions that
are 100s even 1000s of miles from the source of the sulfur
dioxide. Acid rain in Canada could come from sources in
the US, acid rain in Scandinavia came from industrialized areas
in other parts of Europe.
Note at the bottom of the figure above that natural
"pristine" rain is slightly acidic with pH 5.6. This is
because the rain contains dissolved carbon dioxide gas.
Acid rain has a pH less than 5.6.
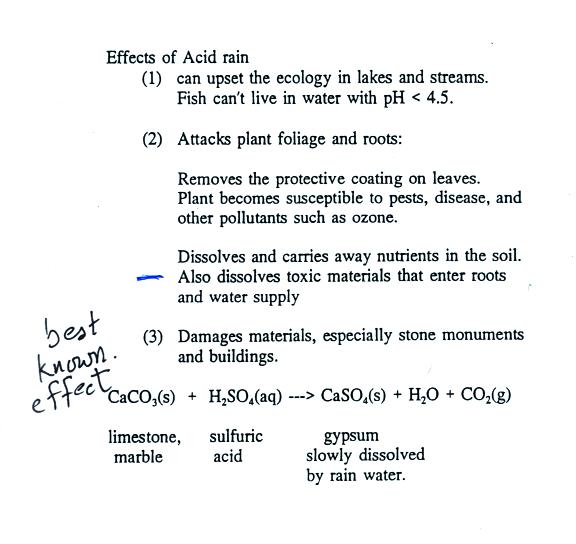
Some of the problems associated with acid rain
are listed above.

|

|
Trees in the Jizera Mountains
(Czech Republic) killed by the effects of acid rain
(source: https://en.wikipedia.org/wiki/Acid_rain)
|
Acid rain damage to the
Ulysses S. Grant Memorial in Washington, D.C. The
sculpture is made of bronze, a mixture of copper and
tin. Copper is dissolved by acid rain and produces
the green stains on the marble base.
(source: https://www.nps.gov/nama/blogs/acid-rains-slow-dissolve.htm)
|

|

|
A chimera
(left, photo source)
and a strix
(right, photo credit: Jawed Karim, photo source)
on Notre Dame Cathedral in Paris. The rounded edges,
grainy texture, and pitting are all characteristic of
damage caused by acid rain. You'll find many more
photographs of Notre Dame gargoyles here.
|
We can fill in another column in our air pollutants chart:
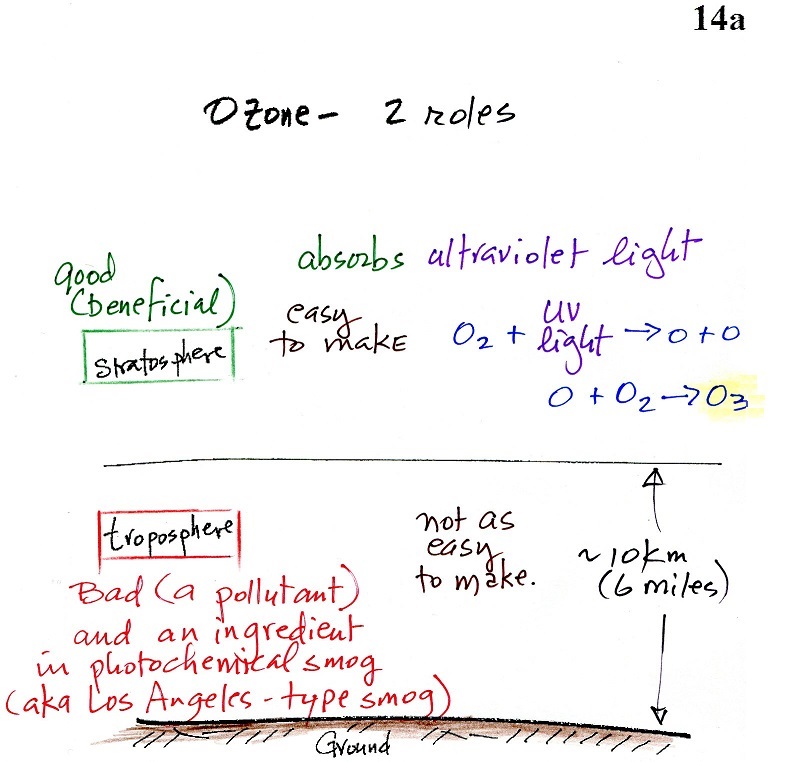
Good (stratospheric) and bad
(tropospheric) ozone
The figure above can be found on page 14a in the photocopied
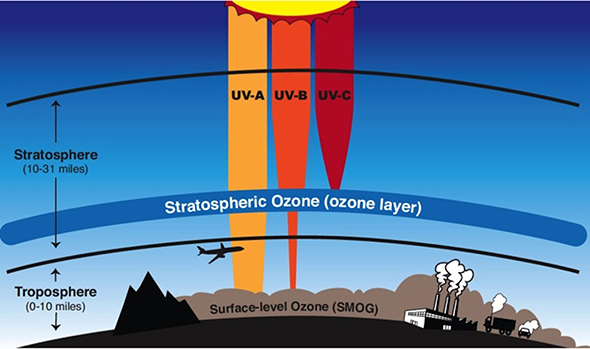
ClassNotes. The ozone layer (ozone in the stratosphere) is
beneficial, it absorbs dangerous high energy ultraviolet light
(which would otherwise reach the ground and cause skin cancer,
cataracts, and actually there are some forms of UV light that
would quite simply kill us).
Here's a nice visual depiction of the role that stratospheric
ozone plays.
Ozone in the troposphere (surface level ozone in the figure
above) is bad, it is toxic and a pollutant. Tropospheric
ozone is also a key component of photochemical smog (also known as
Los Angeles-type smog)
We'll be making some photochemical smog in a class
demonstration. To do this we'll first need some ozone; we'll
make use of the simple stratospheric recipe for making what we
need instead of the more complex tropospheric process (the 4-step
process in the figure below). You'll find more details a
little further down in the notes.
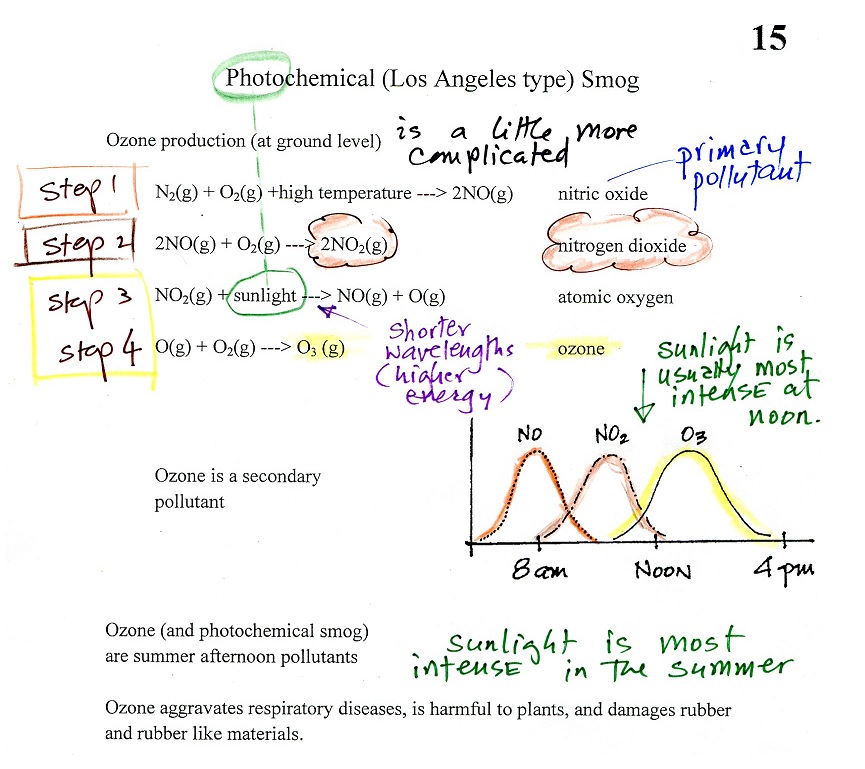
A more complex series of reactions is responsible for the
production of tropospheric ozone. The
production of tropospheric ozone begins with nitric oxide
(NO). NO is produced when nitrogen and oxygen in air are
heated (in an automobile engine for example) and react.
The NO can then react with oxygen in the air to make
nitrogen dioxide, the poisonous brown-colored
gas that I used to make in class.
Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2 react in a 4th step to make ozone (O3) just like happens in the
stratosphere. Because ozone does not come directly from an
automobile tailpipe or factory chimney, but only shows up after
a series of reactions in the air, it is a secondary
pollutant. Nitric oxide (NO) would be the primary
pollutant in this example.
NO is produced early in the day (during the morning rush
hour). The concentration of NO2 peaks somewhat later. Because sunlight is
needed in step #3 and because sunlight is usually most intense
at noon, the highest ozone concentrations are usually found in
the afternoon. Ozone concentrations are also usually
higher in the summer when the sunlight is more intense than at
other times of year.
The American Lung Association's 2019 State of the Air report
mentions that 10 of the 25 cities with the highest tropospheric
ozone concentrations are found in California (Los Angeles is at
the top of the list). Texas has 3 cities in the top 25,
Colorado has two. Arizona (Phoenix), Utah, and Nevada each
have one city. Here's a link to the full
report (167 pages long). Here's a shorter report
with the lists of most
polluted cities. Here a list of the cities in the
United States with the cleanest
air.
Tucson exceeded the EPA NAAQS for ozone for the first time in
August 2018. Phoenix had already exceeded the NAAQS 39 times
that year. Here's a link to the entire
article.

The violation in Tucson, which could
impact the availability of federal transportation funds, is
partly because the allowed ozone concentration is lower than
it used to be. The 80 parts per billion (ppb) 8 hour
average value was lowered to 75 ppb in 2008 and to 70 ppb in
2015.
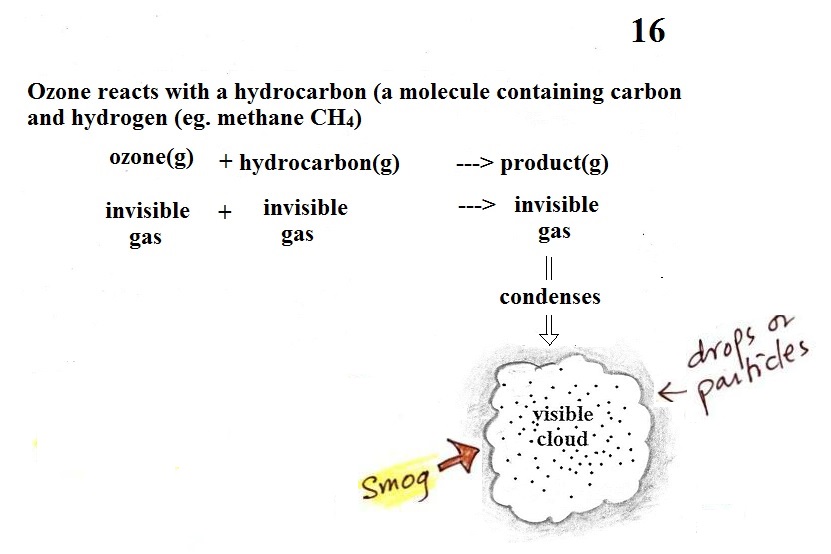
Once ozone is formed, the ozone can react
with a hydrocarbon of some kind to make a product gas. The
ozone, hydrocarbon, and product gas are all invisible, but the
product gas sometimes condenses to make a visible smog cloud or
haze. The cloud is composed of very small droplets or
solid particles. They're too small to be seen but they are
able to scatter light - that's why you can see the cloud.
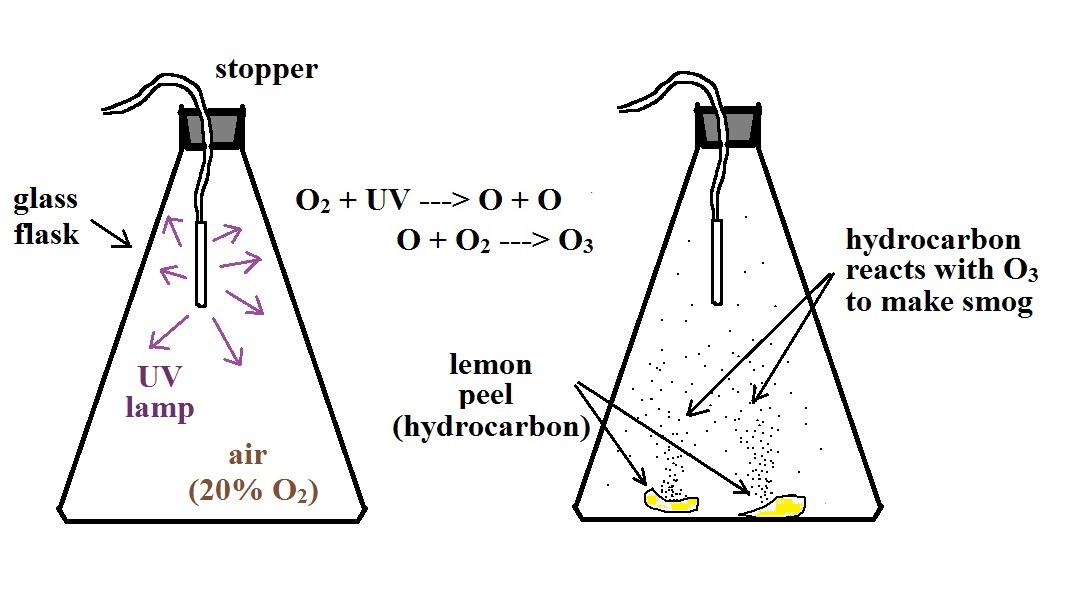
Photochemical smog demonstration
Here's a pictorial summary of the photochemical smog
demonstration.
We started by putting a small "mercury vapor" lamp inside a
flash. The bulb produces a lot of ultraviolet light (the
bulb produced a dim bluish light that we could see, but the UV
light is invisible so we had no way of really telling how
bright the bulb was). The UV light and oxygen in the air
produced a lot of ozone (you could have easily smelled it if
you had taken the cover off the flask).
After a few minutes we turned off the lamp
and put a few pieces of lemon peel into the flash. Part
of the smell of lemon is limonene, a hydrocarbon. The
limonene gas reacted with the ozone to produce a product gas
of some kind. The product gas condensed, producing a
visible smog cloud We shined a laser beam through the
smog cloud to reinforce the idea that we are seeing the cloud
because the drops or particles scatter light.
Here's
a video that I found of a slightly different
version of the demonstration (you really don't miss
much if you don't come to class). Instead of
using UV light to produce the ozone the demonstration
uses an electrical discharge
(the discharge travels from the copper coil inside the
flask to the aluminum foil wrapped around the outside
of the flask). The overall effect is the
same. The discharge splits an oxygen molecule O2
into two oxygen atoms.
O2 + spark ---> O + O
One of the oxygen atoms reacts with an oxygen molecule to form O3
O + O2
---> O3
The smog cloud produced in the video is a little thicker than the
one produced in class. I suspect that is because they first
filled the flask with pure oxygen, 100% oxygen, before making the
ozone. I used air in the room which is 20% oxygen.
More oxygen in the flask means more ozone and a thicker cloud of
Los Angeles type smog.
Here's the nearly completed air pollutants list.
A couple of smog related topics to finish up the
day
Volcanic smog (vog) and laze (lava
and haze)
Volcanic smog can form when the sulfur dioxide (SO2) from an
erupting volcano reacts with oxygen and moisture. The
product of the reaction can form small drops or particles
that begin to scatter light and lower the
visibility.

In this case the hot lava splits water
molecules into hydrogen and oxygen ions. The hydrogen
ions react with chlorine ions from dissociated salt (sodium
chlorida NaCl splits into Na+ and Cl- ions) in the ocean
water. The sudden cooling of lave creates small
fragments of glass. You are left with a haze cloud
consisting of small droplets of hydrochloric acid and
particles of glass. The dangerous haze cloud can cause
skin and eye irritation, lung damage, even death.
We'll cover particulate matter fairly
quickly at the start of our next class.