Thursday Sep. 8, 2016
Some Cajun music before class today from the Lost Bayou
Ramblers "Blue
Moon Special" (6:27) from a concert in Sweden, "Moi J'Connais
Pas" (4:34) from a performance in France.
I'd give you the full period if today's quiz counted for
credit. But it's just for practice so we spent about 30
minutes tying up a loose end and looking ahead to material that we
will be covering next week.
If you weren't in class today you can download a copy of the Practice Quiz and
try answering the questions at home. Then you can have a
look at the answers and
grade your work.
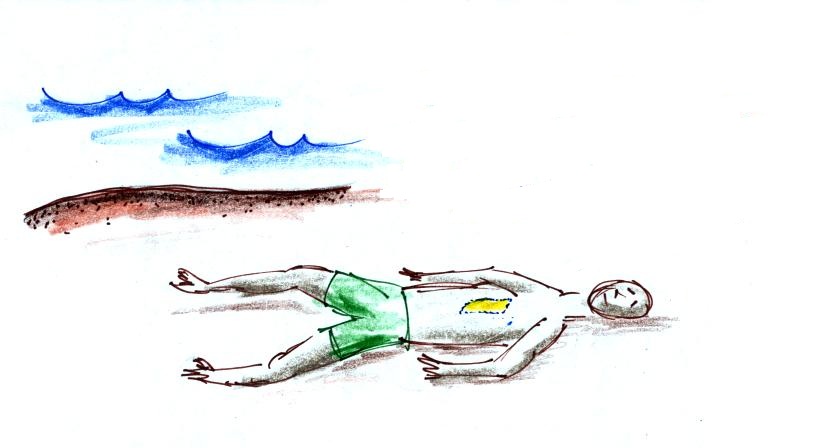
The downward force of air pressure
I am concerning that you might be thinking that a sea
level pressure value of 14.7 psi might not sound like
much. But when you start to multiply 14.7 by all
the square inches on your body it turns into a lot of pounds
of force.
The yellow box on the person's chest in the picture is a brick
size, 4" x 8" = 32 square inch, area. If you multiply 14.7
psi by 32 sq. in. you get 470 pounds! It would take a
stack of 90 to 100 bricks to produce that much weight.
Imagine lying on the beach with 90 bricks stacked up on your
chest. That's what the atmosphere is doing.
Why isn't the person in the picture above
crushed by the weight of the atmosphere above. The
answer is that the person's body pushes back with the same
amount of force. Air does the same thing. This
is the topic we will explore next week.
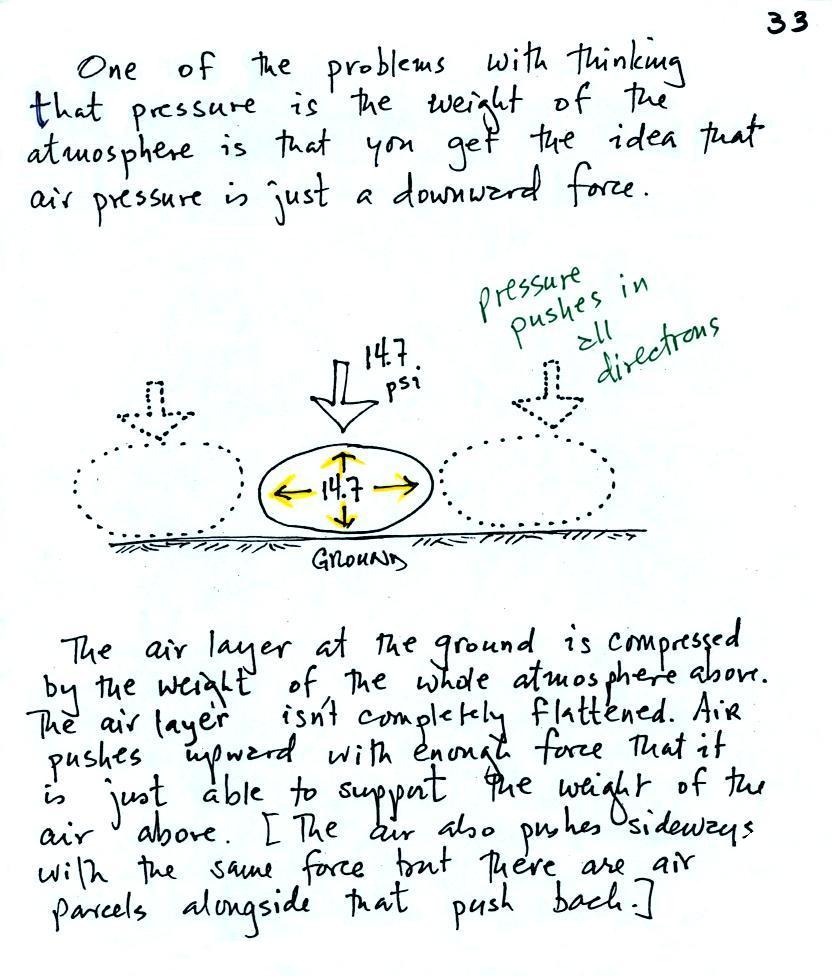
The upward (and sideways) force of air pressure
Air pressure is a force that pushes
downward, upward, and sideways. If you fill a balloon with
air and then push downward on it, you can feel the air in the
balloon pushing back (pushing upward). You'd see the air in
the balloon pushing sideways as well.
We would be able to see this by placing a
brick on top of a balloon. The balloon gets squished
(pushed out sideways) but not flattened. It eventually
pushes upward with enough force to support the brick. The
squished balloon is what air at the bottom of the atmosphere
looks like. And it is supporting more than just
one brick, it is supporting a pile 90 to 100 bricks tall (just
like the yellow box on the chest of the guy at the beach).
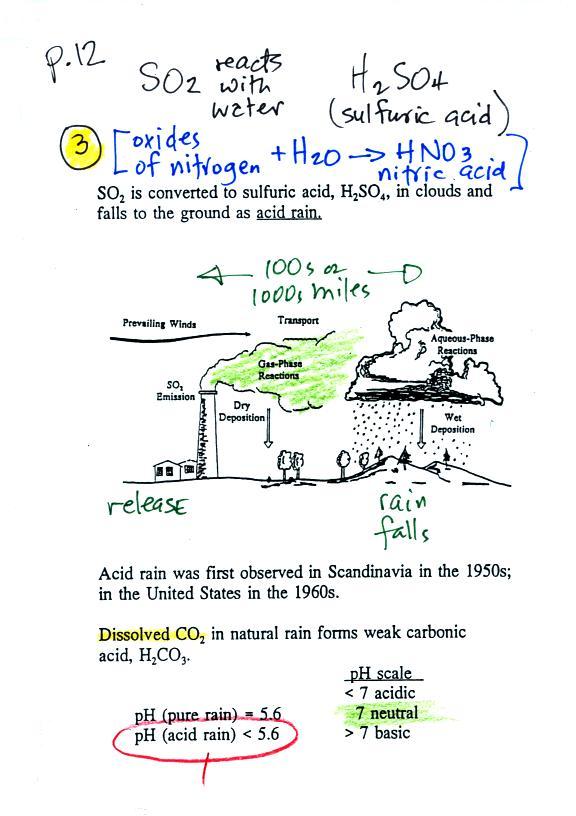
Acid rain
Sulfur
dioxide is one of the pollutants that can react with
water in clouds to form acid rain (some of the
oxides of nitrogen can also react with water to form
nitric acid). The formation and effects of
acid rain are discussed on p. 12 in the photocopied
Class Notes.
Acid rain is often a problem in regions that
are 100s even 1000s of miles from the source of the sulfur
dioxide. Acid rain in Canada could come from sources in
the US, acid rain in Scandinavia came from industrialized areas
in other parts of Europe.
Note at the bottom of the figure above that natural
"pristine" rain has a pH less than 7 and is slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. The acid rain demonstration described below
and done in class should make this point clearer.
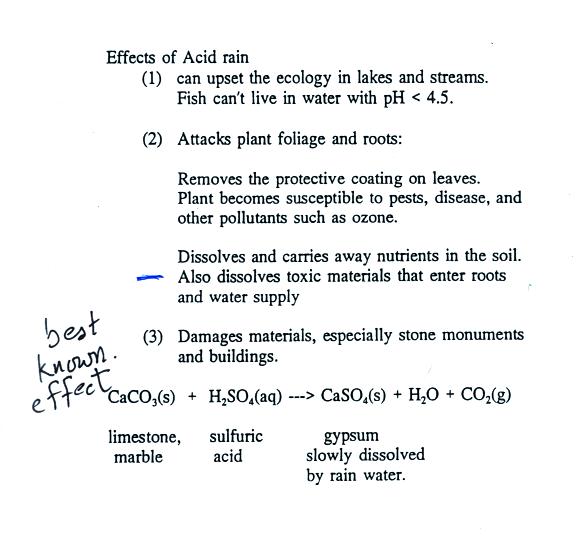
Some of the problems associated with acid rain are
listed above.
Acid Rain Demonstration
Some common acids are listed below. In
solution the acid molecules dissociate (split) into pieces.
The presence of H+ ions is what makes these materials
acids.
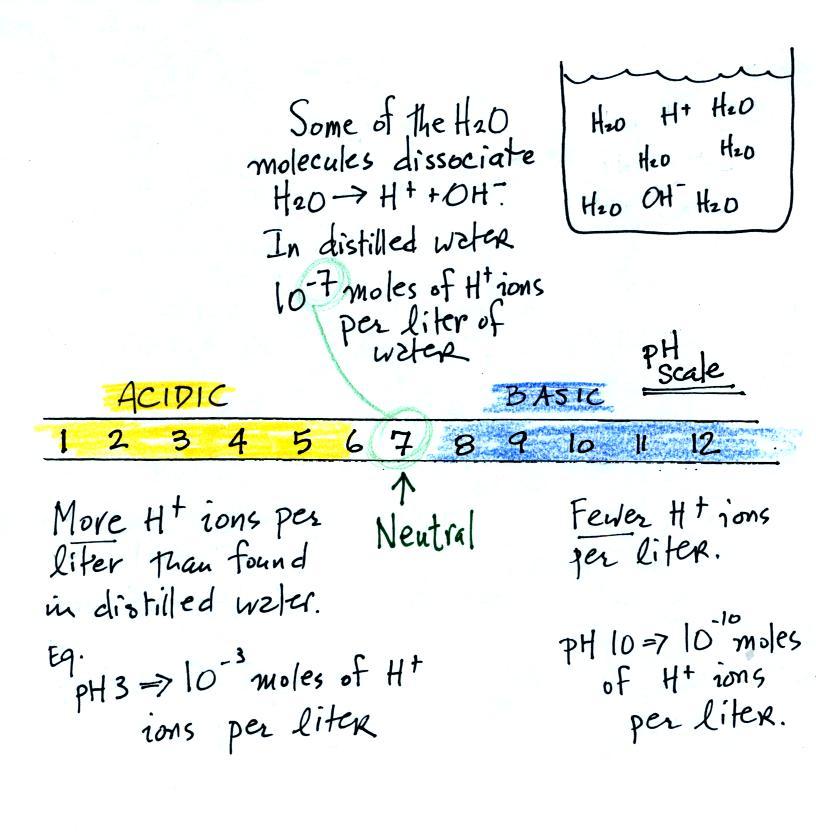
And
actually it isn't enough to just have H+ ions for
something to be an acid. There are H+
ions in pure distilled water and it's not an acid. To be an
acid the H+ ion concentration must be
greater than is found in distilled water. The H+
ion concentration in distilled water is 10-7 moles
of H+ ions per liter of
water. A mole is
just a number, a very large number (6 x 1023).
It's the same idea as dozen. A dozen means you've got 12 of
something. 10-7 moles
of H+ ions per liter is
10-7 times
6 x 1023 = 6 x 1016
H+ ions per liter of water.
The pH scale
We often use the pH scale to measure acid concentration. An
H+ ion concentration of 10-7 moles/liter corresponds to
pH 7 (the pH value is computed by taking the -log10
of the H+
ion concentration). Other than remembering the pH
value of distilled water is pH7, these are all details
you don't need to worry about.
It is also possible to have fewer H+
ions in a solution than would be found in distilled water. A
solution like this is basic.
Pouring some acid into water would increase the H+ ion concentration (from 10-7moles/liter to 10-3moles/liter, perhaps as shown
in the example above). Adding a base to water will decrease
the H+ ion
concentration (from 10-7moles/liter
to 10-10moles/liter,
perhaps).
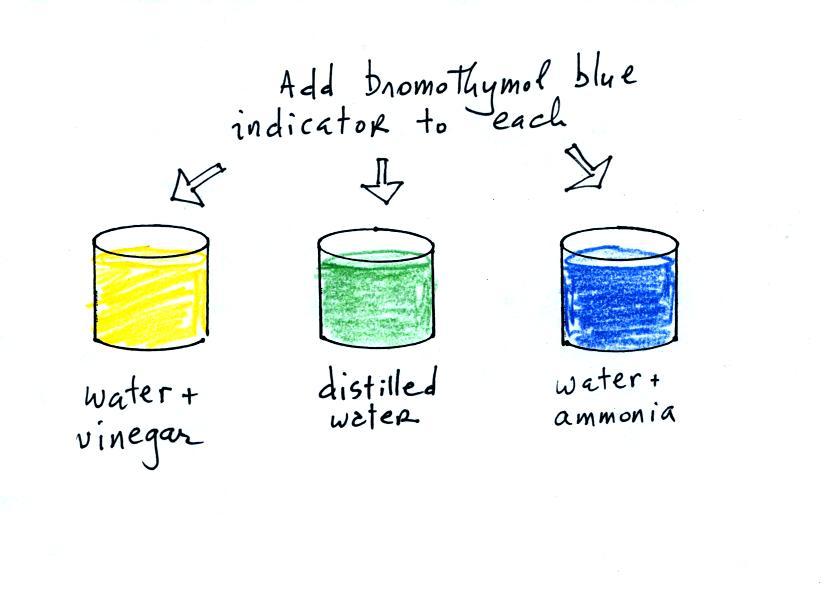
Now we can proceed with the demonstration. We will start
with three 1000 mL beakers each filled with distilled water.
Some vinegar (contains acetic acid) was added to the left beaker.
Some ammonia (a base) was added to the right beaker.
Acid/Base indicator solution
Then we added some bromothymol blue,
a color indicator solution, to all three beakers.
Bromothymol blue has the amazing property of changing color
depending on whether it is mixed with an acid (golden yellow) or a
base (deep blue).
So far we have just reviewed the pH scale and introduced
acid/base indicator solutions.
When sulfur dioxide is released into the air it reacts with the
water in clouds to produce acid rain. I really can't use SO2
in class because it's poisonous. I'll use carbon dioxide, CO2,
instead.
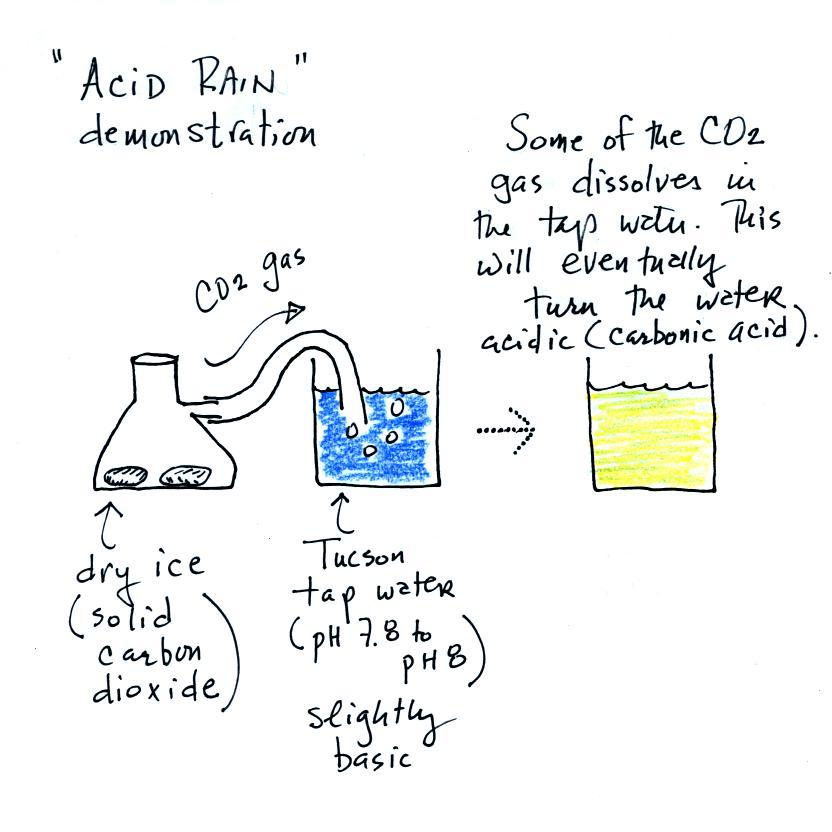
We added some Tucson tap water to a large 2000 mL beaker.
This represents a cloud. We added some bromothymol
blue to the tap water and it turned blue. So we know that
Tucson tap water is basic.
A few small pieces of dry ice are put into a flask. We close
the flask with a stopper. The end of a piece of tubing
connected to the flask is immersed in the tap water.
Dry ice sublimes. It turns directly from solid to ice
(ordinary ice melts and turns from solid to liquid). The
gaseous CO2 is invisible but
you can tell it is there because of the bubbles in the tap
water. Some of the CO2
dissolves as it bubbles through the water and slowly turns the
water acidic. You can tell that this is occurring because
the bromothymol blue indicator turns from deep blue to green and
eventually to yellow.
I call this a "sort of" acid rain demonstration. That's
because we haven't really produced acid rain. Air contains
carbon dioxide and the CO2 makes natural rain slightly acidic (pH5.6 or
so). To make true acid rain we would need a different gas,
something other than carbon dioxide, something that would lower
the pH below 5.6.
While we didn't actually produce acid rain, there is concern
that increasing atmospheric concentrations of carbon dioxide will
dissolve and acidify the world's oceans. This is discussed
in the following article from The Christian Science Monitor.
You can download a copy of the article here.
The main concern over increasing atmospheric carbon dioxide
concentrations is global warming from enhancement of the
greenhouse effect. We will discuss this topic at some point
during the semester.
Carbonated beverages contain dissolved carbon dioxide and are
acidic. Soft drinks also contain phosphoric acid which makes
them even more acidic than the dissolved carbon dioxide would
do. With time the acidity of soft drinks can damage tooth
enamel.