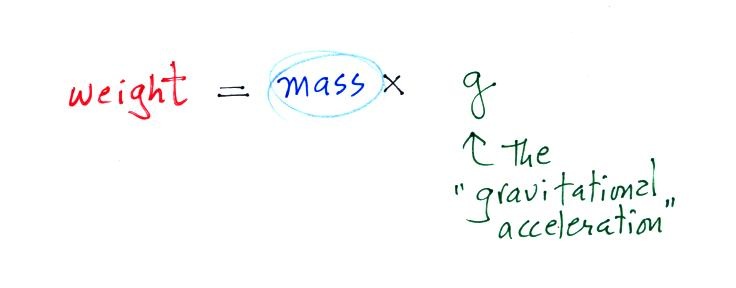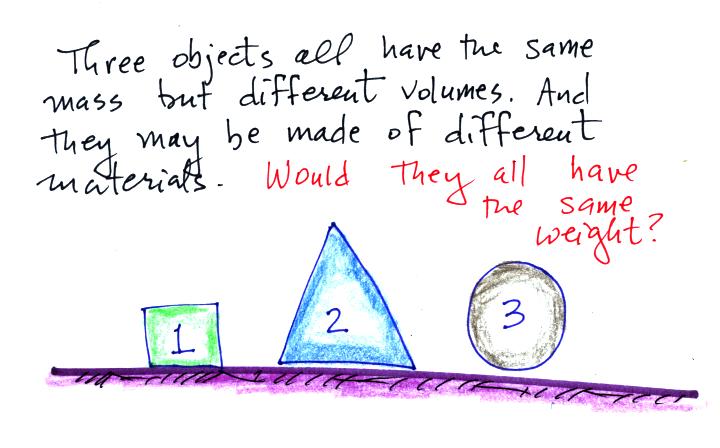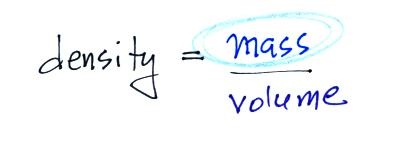Dave
McGraw and Mandy Fer: "Seritony"
(4:52), "Cat
Creek" (5:52), "Grow"
(4:17), "Comin
Down" (5:12)
The
1S1P
Assignment #1a
reports were
collected
today.
It takes some
time to grade
this kind of
work.
You might hope
(best case
scenario) to
get them back
about this
time next
week. I
have decided
on a due date
for Assignment
#1b the
Scattering of
Light
reports.
The due date
is Tue., Sept.
20.
That's two
weeks from
today and is
the Tuesday
before Quiz
#1, also the
day the
Experiment #1
reports are
due.
The In-class
Optional
Assignment
from last
Thursday was
returned
today.
If you don't
see a grade
marked on the
top of your
paper you
received full
credit (0.2%
of extra
credit).
Here are answers
to the
questions
on the
assignment.
You'll also
find your
personal class
ID marked on
your paper
near your
name.
This letter
& number
ID is what I
will use when
I post class
grades
online.
The main event
in the next 24
hours or so
will be the
approach and
passage of
Hurricane
Newton.
The current
forecast path
has the storm
passing very
near Tucson
tomorrow.
It
will have
weakened a lot
by the time it
gets near
Tucson but
still could
produce in a
lot of rain.
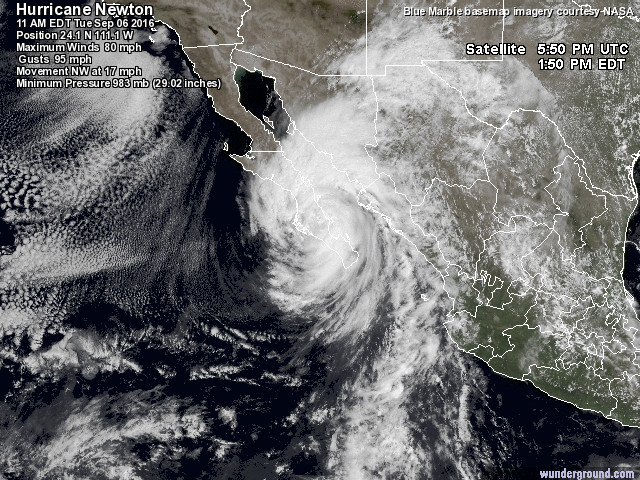
|

|
Satellite photograph of Hurricane Newton at
11 am MST (I think). Image from a Weather
Underground page.
|
Predicted path of Hurricane Newton.
Issued by the National
Hurricane Center at 2 pm MST. The storm is
expected to cross the Mexico - Arizona border as a tropical
storm so we might
experience some windy conditions. There is also a
chance of heavy rainfall Wednesday.
|
We're starting a new topic:
Mass, weight, density, and pressure.
Pressure
especially is a pretty important concept.
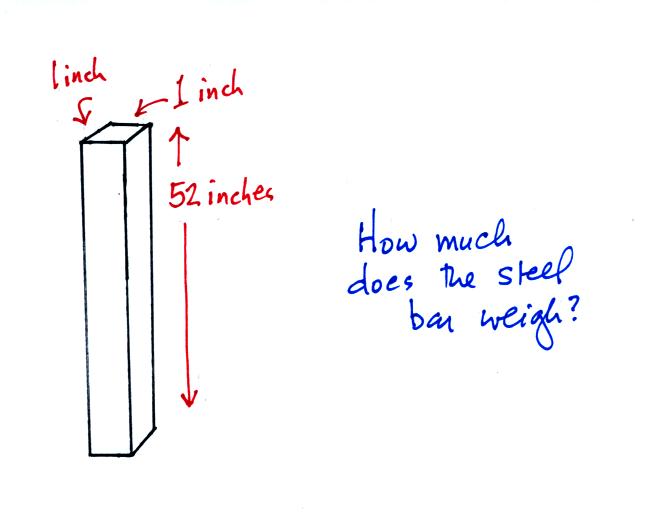
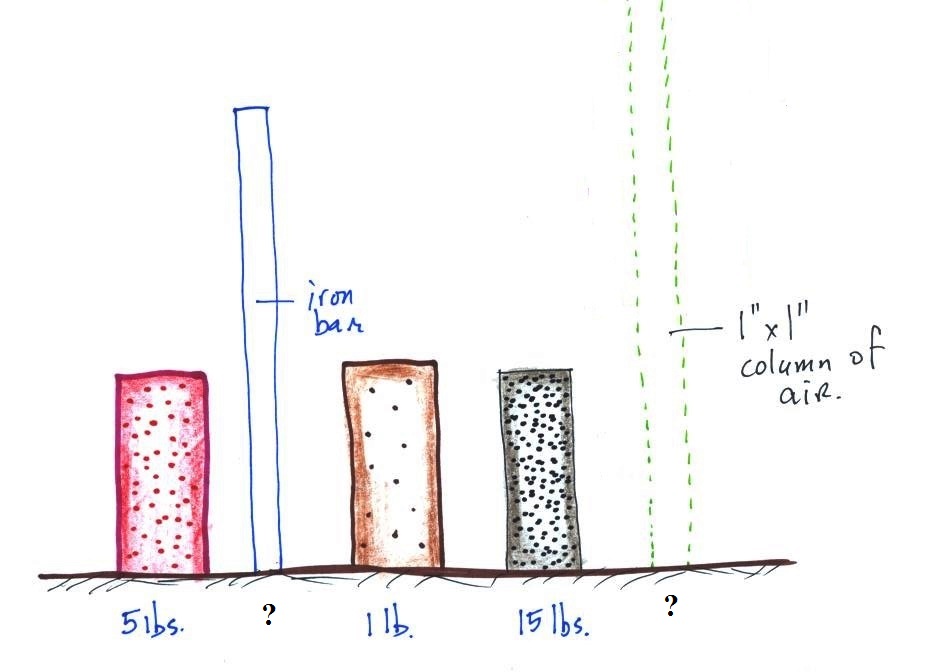
Weight is something you can feel. I'll
pass an iron bar around in class (it's
sketched below) - lift it and try to guess or
estimate it's weight. The fact that it
is a 1" by 1" is significant. More about
the bar later in today's notes.
I used to
pass around a couple of small plastic bottles (see
below). One contained some water, the other an equal
volume of mercury (here's the source
of the nice photo of liquid mercury below at
right). I wanted you to appreciate how much
heavier and denser mercury is compared to
water.
But the plastic bottles have a way of getting brittle with
time and if the mercury were to spill in the classroom the
hazardous material people would need to come in and clean it
up. That would probably take a lot of time and would be
very expensive. So this semester I'll pass around a
smaller, much safer, sample of mercury so that you can at
least see what mercury it looks like (it's a recent purchase
from a company in
London). I'll keep the plastic bottles of mercury
up at the front of the room just in case you want to see how
heavy the stuff is.
It
isn't so much the liquid mercury that is a hazard, but
rather the mercury vapor. Mercury vapor is used in
fluorescent bulbs (including the new energy efficient CFL
bulbs) which is why they need to be disposed of
carefully. That is a topic that will come up again
later in the class.
Mercury and bromine are the only
two elements that are found naturally in liquid
form. All the other elements are either gases or
solids.
I am hoping that you will remember and understand the
following statement
atmospheric
pressure at any level in the atmosphere
depends on (is determined by)
the weight
of the air overhead
We'll
first review the concepts of mass, weight, and density
but understanding pressure is our main goal.
I've numbered the various sections (there are a total
of 8) to help with organization. There's also a
summary at the end of today's notes.
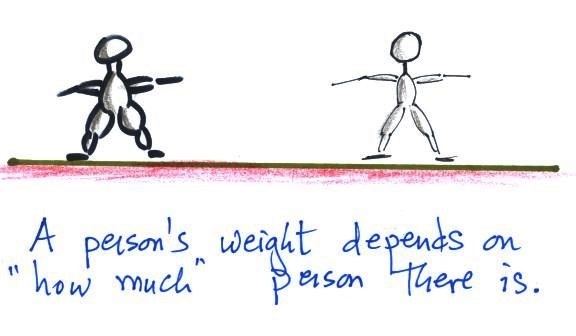
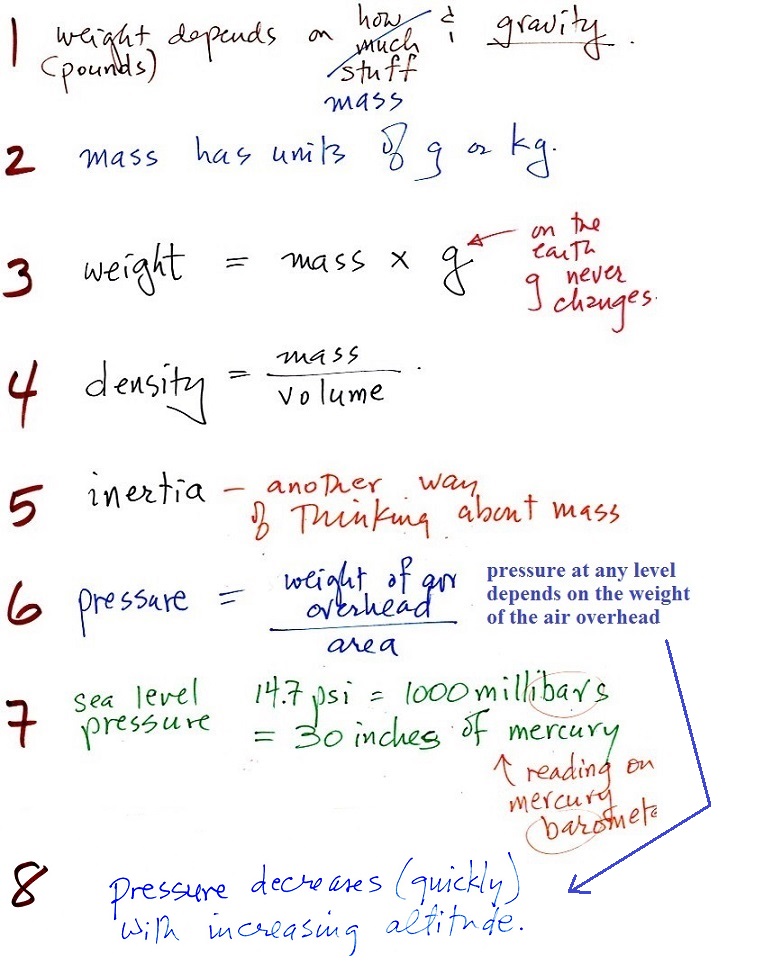
1.
weight
This is a good place to start because this is
something we are pretty familiar with. We
can feel weight and we routinely measure weight.
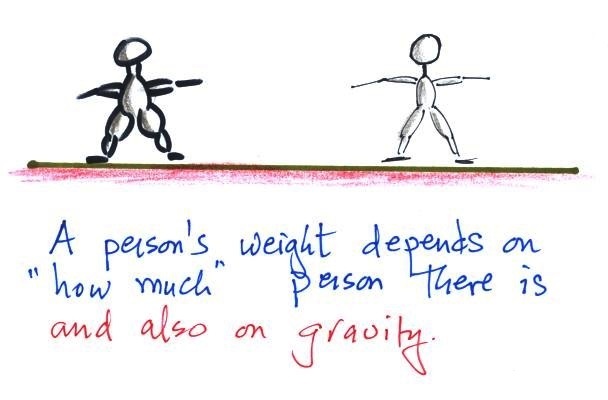
A person's weight also depends
on something else.
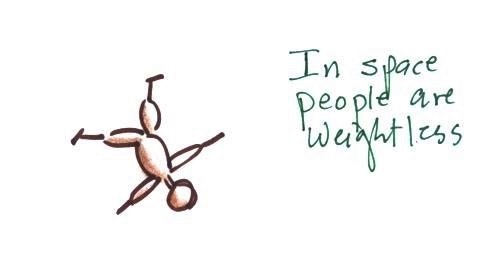
In outer space away from
the pull of the earth's gravity people are weightless.
Weight depends on the person and on the pull of
gravity.
We
measure weight all
the time. What
units do we
use? Usually
pounds, but
sometimes ounces or
maybe tons. A
student will
sometimes mention
Newtons, those are
metric units of
weight (force).
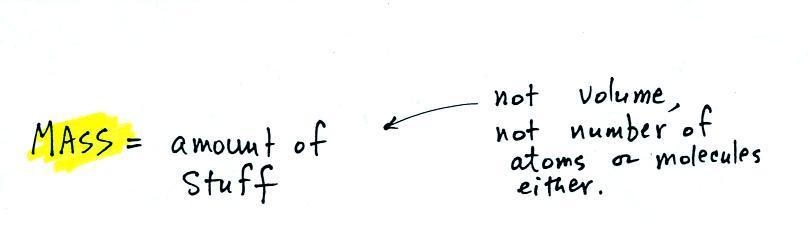
2. mass
Rather than just saying the
amount of something it is probably better to use the
word mass
It would be possible to have equal volumes of
different materials, with the same total number of atoms or
molecules, and still have different masses.
Grams (g) and kilograms (kg) are commonly used units of mass (1
kg is 1000 g).
3. gravitational
acceleration
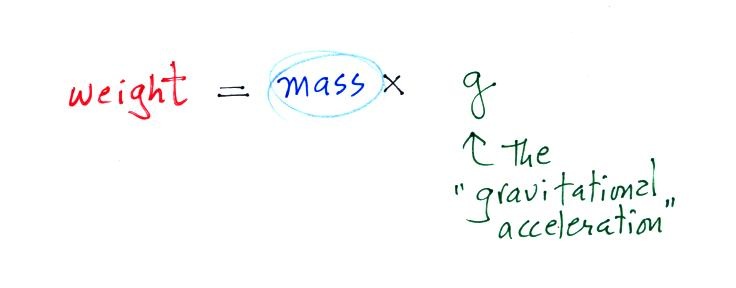
On the surface of the earth, weight is
mass times a constant, g, known as the
gravitational acceleration. The value of g
is what tells us about the strength of gravity on the earth;
it is determined by the size and mass of the earth. On
another planet the value of g would be
different. If you click here
you'll find a little (actually a lot) more information about
Newton's Law of Universal Gravitation. You'll see how
the value of g is determined and why it is called
the gravitational acceleration. These aren't details
you need to worry about but they're there just in case
you're curious.
Here's a question to test your understanding.
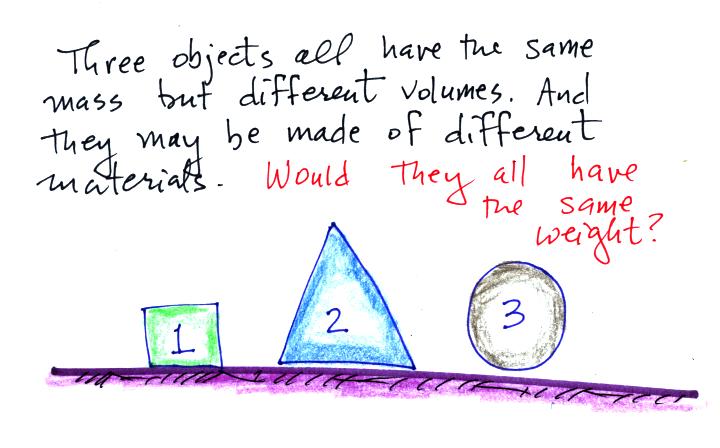
The masses are all the same. On the earth's
surface the masses would all be multiplied by the same value of g.
The weights would all be equal. If all 3 objects
had a mass of 1 kg, they'd all have a weight of 2.2 pounds.
That's why we can use kilograms and pounds interchangeably.
The following figure show a situation where two
objects with the same mass would have different weights.
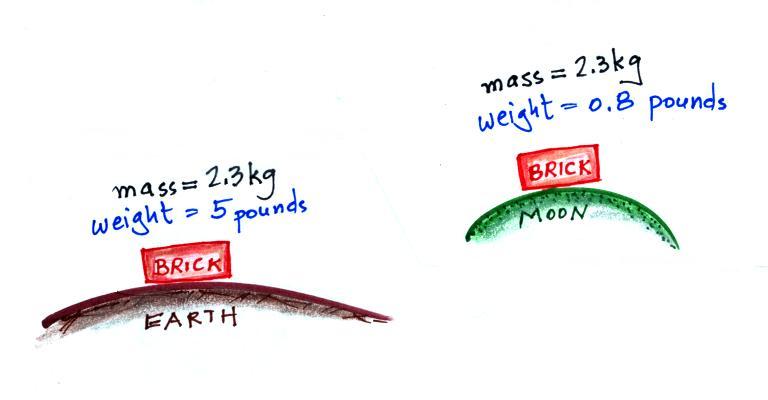
On the earth a brick has a mass of about
2.3 kg and weighs 5 pounds. If you were to travel to the
moon the mass of the brick wouldn't change (it's the same
brick, the same amount of stuff). Gravity on the moon is
weaker (about 6 times weaker) than on the earth because the
moon is smaller, the value of g on the moon is
different than on the earth. The brick would only weigh
0.8 pounds on the moon. The brick would
weigh almost 12 pounds on the surface on Jupiter where
gravity is stronger than on the earth.
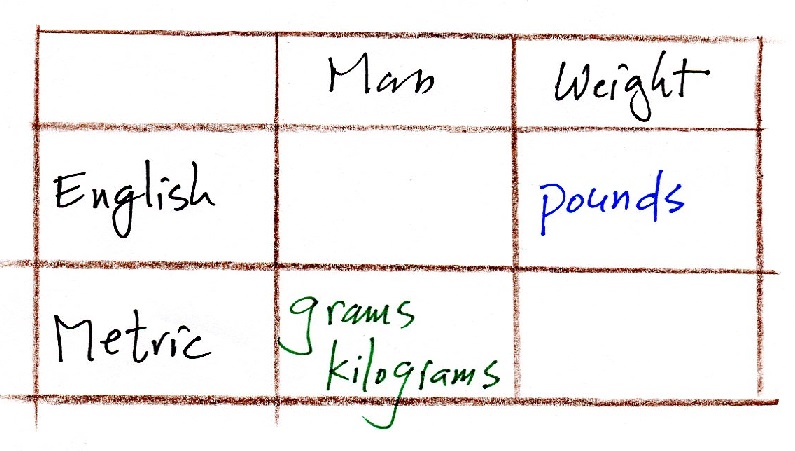
Any idea what the English units for
mass and the Metric units for weight (force) are?
"Slugs" if you can believe it are the English units for
mass. The metric units for weight (force) are dynes (if
mass is in grams) or Newtons (for mass in kilograms)
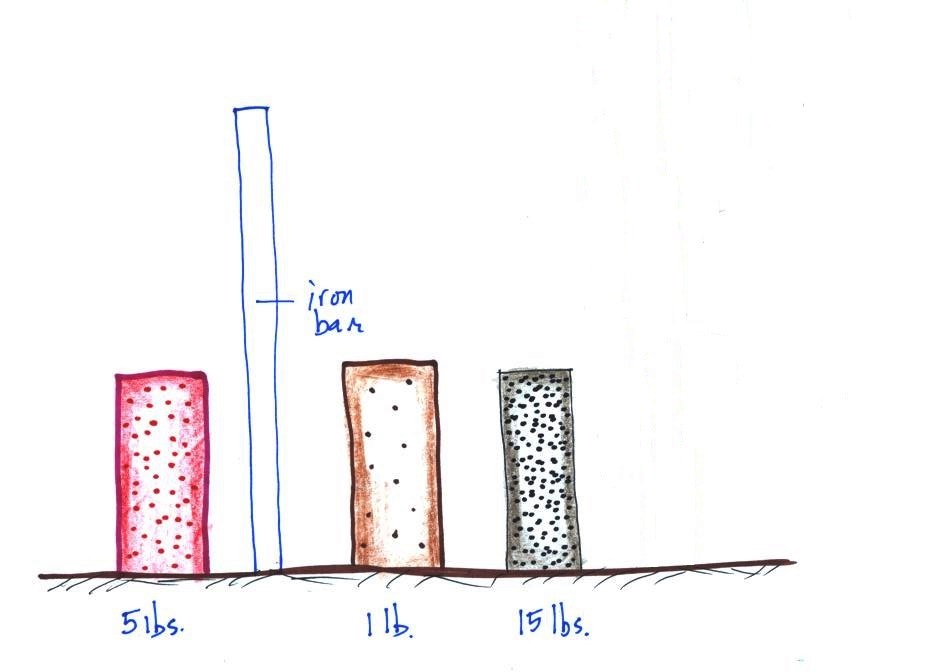
The
easiest way to determine which is which is to lift each
one. One of them weighed about 1 pound (wood), the 2nd
about 5 pounds (a brick) and the last one was 15 pounds (a
block of lead).
The point of all this was to get you thinking about
density. Here we had three objects of about
the same size with very different weights. Different
weights means the objects have different masses (since weight
depends on mass). The three different masses, were
squeezed into roughly the same volume producing objects of
very different densities.
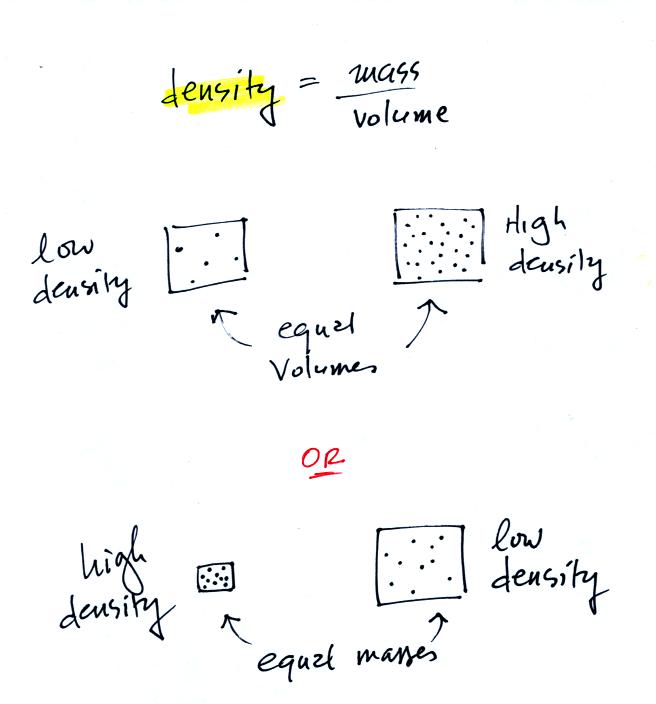
4. density
The brick is in the back, the lead
on the left, and the piece of wood (redwood) on the right.
The wood is less dense than water (see the table below) and
will float when thrown in water. The brick and the lead
are denser than water and would sink in water.
We'll be more concerned with air in this
class than wood, brick, or lead.
In the first example
below we have two equal volumes of air but the amount in
each is different (the dots represent air
molecules).
The amounts of air (the masses) in the second example are the
same but the volumes are different. The left example
with air squeezed into a smaller volume has the higher
density.
material
|
density g/cc
|
air
|
0.001
|
redwood
|
0.45
|
water
|
1.0
|
iron
|
7.9
|
lead
|
11.3
|
mercury
|
13.6
|
gold
|
19.3
|
platinum
|
21.4
|
iridium
|
22.4
|
osmium
|
22.6
|
g/cc = grams per cubic centimeter
cubic centimeters are units of volume - one cubic
centimeter is about the size of a sugar cube
1 cubic centimeter is also 1 milliliter (mL)
I would like to get my hands on a brick-size piece of
iridium or osmium just to be able to feel how heavy it
would be - it's about 2 times denser than lead.
Here's a more subtle concept. What if we were
in outer space with the three wrapped blocks of lead, wood, and
brick? They'd be weightless.
Could we tell them apart then? They would still have very
different densities and masses but we wouldn't be able to feel how
heavy they were.
5.
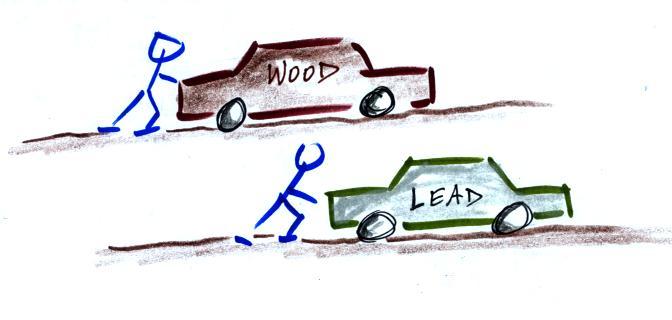
inertia
I think the following illustration will
help you to understand inertia.
Two stopped cars. They are the same size except
one is made of wood and the other of lead. Which
would be hardest to get moving (a stopped car resists
being put into motion). It would take considerable
force to get the lead car going. Once the cars are
moving they resist a change in that motion. The
lead car would be much harder to slow down and stop.
This is the way you could try to distinguish
between blocks of lead, wood, and brick in outer space.
Give them each a push. The wood would begin moving more
rapidly than the block of lead even if both are given
the same strength push.
I usually
don't mention in class that this concept of
inertia comes from Newton's 2nd law of motion
F = m a
force = mass x acceleration
We can rewrite the equation
a = F/m
This shows cause and effect more clearly. If you exert a
force (cause) on an object it will accelerate (effect).
Acceleration can be a change in speed or a change in direction (or
both). Because the mass is in the denominator, the
acceleration will be less when mass (inertia) is large.
Here's where we're at
From left to right the brick, the iron bar, the piece
of wood, and the lead block. The weight of the iron bar
is still unknown.
Now
we're close to
being ready to
define (and
hopefully
understand)
pressure.
It's a pretty
important
concept.
A lot of what
happens in the
atmosphere is
caused by
pressure
differences.
Pressure
differences
cause
wind.
Large pressure
differences
(such as you
might find in
a tornado or a
hurricane) can
create strong
and
destructive
storms.
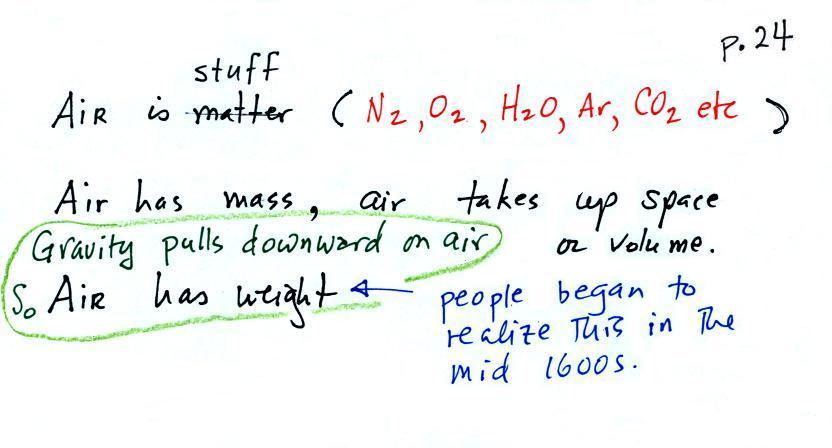
The air that
surrounds the earth has mass. Gravity pulls downward on
the atmosphere giving it weight. Galileo conducted a
simple experiment to prove that air has weight (in the
1600s).
We
could add a very
tall 1 inch x 1
inch column of air
to the
picture.
Other than being a
gas, being
invisible, and
having much lower
density it's
really no
different from the
other objects.
6. pressure
Atmospheric pressure at
any level in the atmosphere
depends on (is determined
by)
the weight of the air
overhead
This
is one way, a sort of large, atmosphere
size-scale way, of understanding air pressure.
Pressure depends on, is determined by, the weight of the
air overhead. To determine the pressure you need to
divide by the area the weight is resting on.
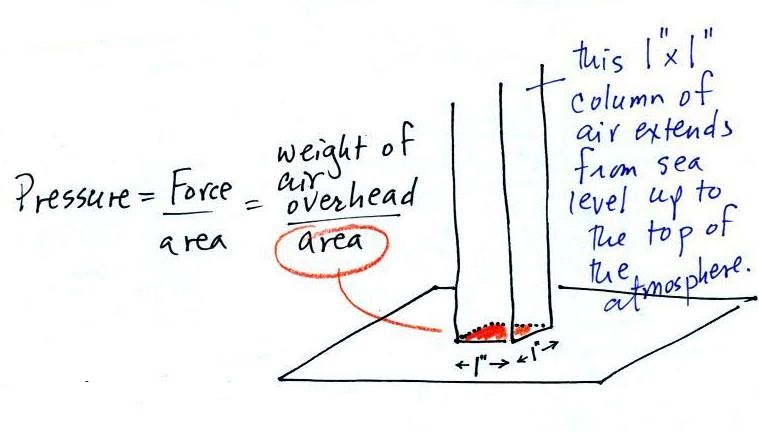
and here we'll apply the
definition to a column of air stretching from sea
level to the top of the atmosphere (the figure below
is on p. 24 in the ClassNotes)
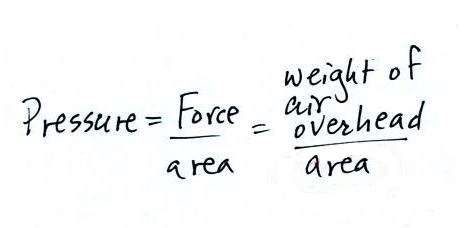
Pressure is defined as force divided by area. Atmospheric
pressure is the weight of the air column divided by the area at
the bottom of the column (as illustrated above).
Under normal conditions a 1 inch by 1 inch column of air
stretching from sea level to the top of the atmosphere will weigh
14.7 pounds.
Normal atmospheric pressure at sea level is 14.7 pounds per square
inch (psi, the units you use when you fill up your car
or bike tires with air).
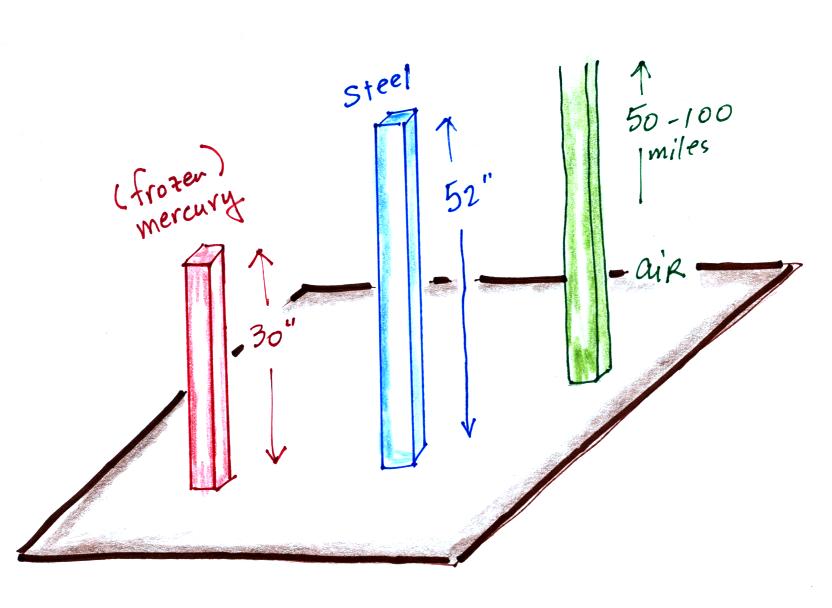
Now back to the iron bar. The bar actually weighs
14.7 pounds (many people I suspect think it's heavier than
that). When you stand the bar on end, the pressure at
the bottom would be 14.7 psi.
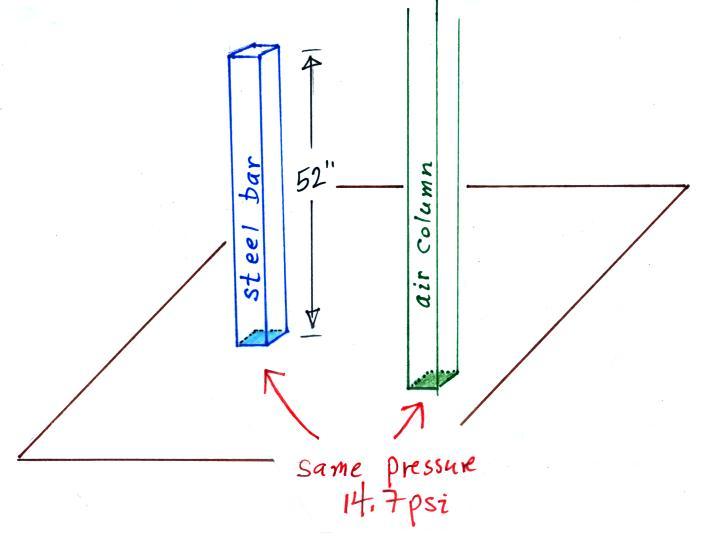
The weight of the 52 inch
long 1" x 1" steel bar is the same as a 1" x 1" column
of air that extends from sea level to the top of the
atmosphere 100 or 200 miles (or more) high. The
pressure at the bottom of both would be 14.7 psi.
7. pressure units
Pounds per square inch, psi, are
perfectly good pressure units, but they aren't the ones
that meteorologists use most of the time.
Typical sea
level pressure is 14.7 psi or about 1000 millibars
(the units used by meteorologists and the units that we will
probably mostly use in this class) or about 30 inches of
mercury. Milli means 1/1000 th. So
1000 millibars is the same as 1 bar. You sometimes see
typical sea level pressure written as 1 atmosphere.
Inches
of mercury refers to the reading on a mercury
barometer. This seems like unusual
units for pressure. But if you remember the chart
earlier, Mercury (13.6 grams/cm3)
is denser than steel ( about 7.9 grams/cm3 ). If we could some
how construct a 1" x 1" bar of mercury it would only need to
be 30 inches long to equal the weight or the iron bar or the
weight of a tall column of air.
Each of these columns would weigh 14.7 pounds. The
pressure at the base of each would be the same.
A mercury barometer is, we'll find, just a balance.
You balance the weight of a very tall column of air with the
weight of a much shorter column of (liquid) mercury.
Someone asked about the freezing point of
mercury in a previous class. It's not all that cold -38
C which is about -39 F. Alcohol is often used in
thermometers in cold locations to avoid having the mercury
freeze (and break the thermometer).
You never know where something you learn in ATMO 170 will
turn up. Take pressure units for example.
I once lived and worked in France. Here's a picture of a
car I owned when I was there (the one below is in mint
condition, mine was in far worse shape).
It's
a Peugeot
404. I was at
the service station one day and decided to pump up the
tires a little bit. I wanted to put about 30 psi
into the tires but the scale on the compressor only went
up to 4. Not knowing much French it took me 15
minutes, before I realized
the air compressor was marked in "bars" not "psi". Since
14.7 psi is about 1 bar, 30 psi would be about 2 bars (90 psi
needed in my bicycle tires would be about 6 bars).
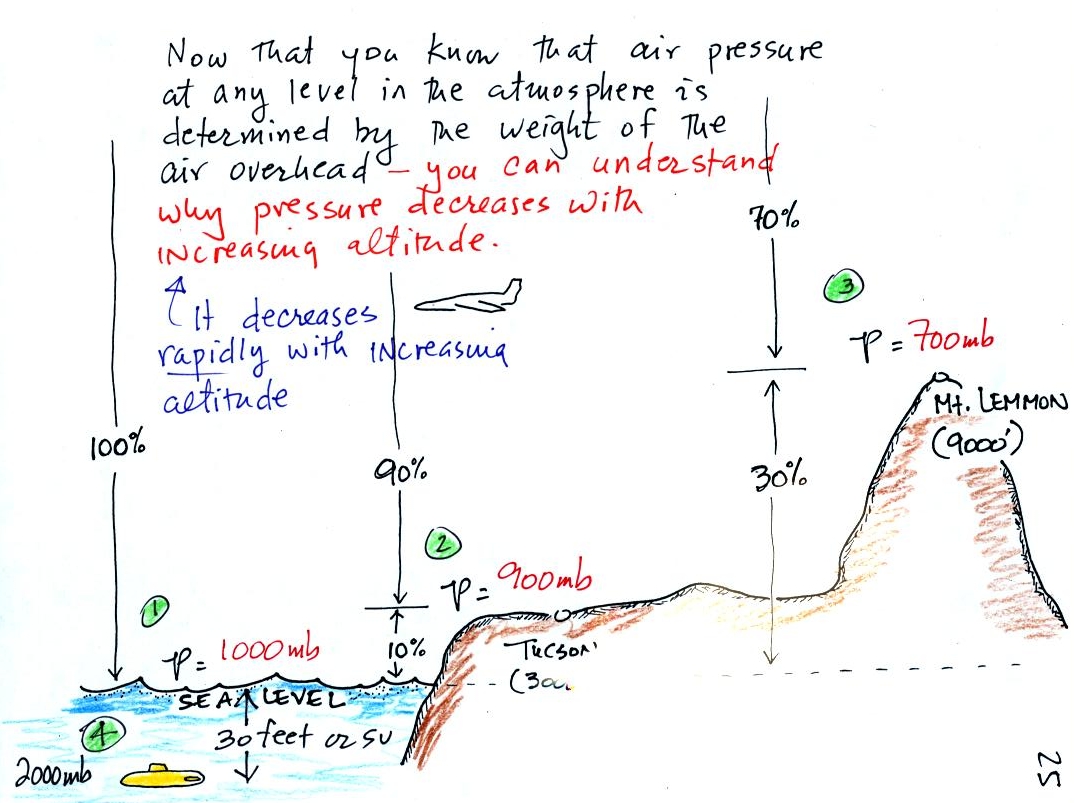
8. changes in atmospheric pressure with altitude
If you remember and understand the statement
atmospheric
pressure at any level in the atmosphere
depends on (is determined by)
the weight
of the air overhead
You can quickly and easily figure out what happens to air
pressure as you move upward in the atmosphere. A
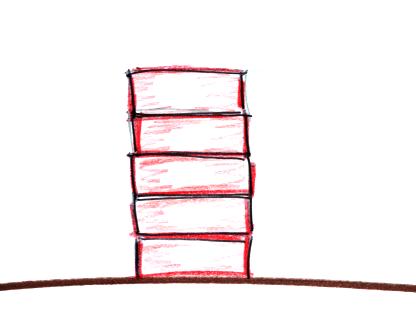
pile of bricks is helpful too. Here's
a picture of 5 bricks stacked on top of each
other.
Each brick weighs 5 pounds, there's a total of 25
pounds of weight. At
the bottom of the pile you would measure a weight of 25
pounds. If you moved up a brick you would measure a
weight of 20 pounds, the weight of the four bricks that
are still above. The pressure would be less.
Weight and pressure will decrease as you move up the pile.
Layers
of air in the atmosphere is not too much
different from a pile of bricks.
Pressure at any level is determined by the
weight of the air still overhead.
Pressure decreases with increasing
altitude because there is less and less
air remaining overhead.
At sea level altitude, at Point 1, the pressure
is normally about 1000 mb. That is determined by the
weight of all (100%) of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000 feet
above sea level (most of central Tucson is a little lower
than that around 2500 feet). At 3000 ft. about 10%
of the air is below, 90% is still overhead. It is
the weight of the 90% that is still above that determines
the atmospheric pressure in Tucson. If 100% of the
atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at
the summit of Mt. Lemmon (9000 ft. altitude at Point 3) because 70%
of the atmosphere is overhead..
Pressure decreases rapidly with
increasing altitude. We will find that pressure
changes more slowly if you move horizontally.
Pressure changes about 1 mb for every 10 meters of
elevation change. Pressure changes much more slowly
normally if you move horizontally: about 1 mb in 100
km. Still the small horizontal changes are what
cause the wind to blow and what cause storms to form.
Point
4 shows a submarine at a depth of about 30 ft.
or so. The pressure there is determined by the
weight of the air and the weight of the water
overhead. Water is much denser and much heavier than
air. At 30 ft., the pressure is already twice what
it would be at the surface of the ocean (2000 mb instead
of 1000 mb).
I learned about a relatively new sport called free diving
a semester or two ago. Basically divers see how deep they
can go while holding their breath. They must descend and
return to the surface on just a single lungful of air. It is
a very hazardous sport. Here is a link
to an article about a diver that made it to a depth of 236
feet but died upon reaching the
surface. Death was caused by the
high pressure deep under water forcing fluid from the
blood into the diver's lungs.
As promised, here's a short summary of the main points from the
mass, weight, density, and pressure section.