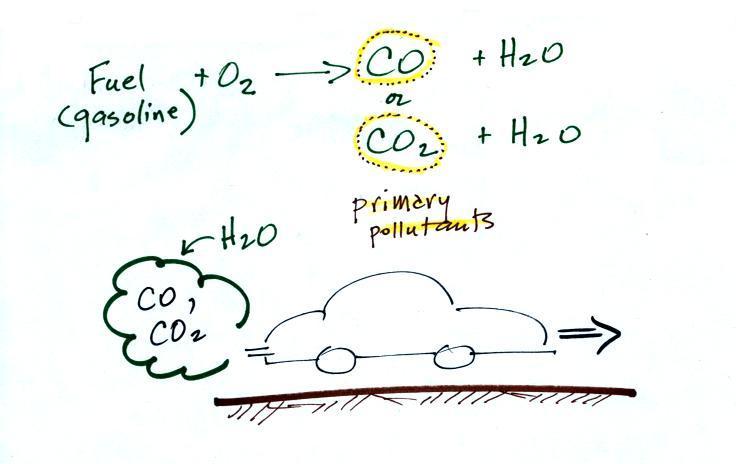
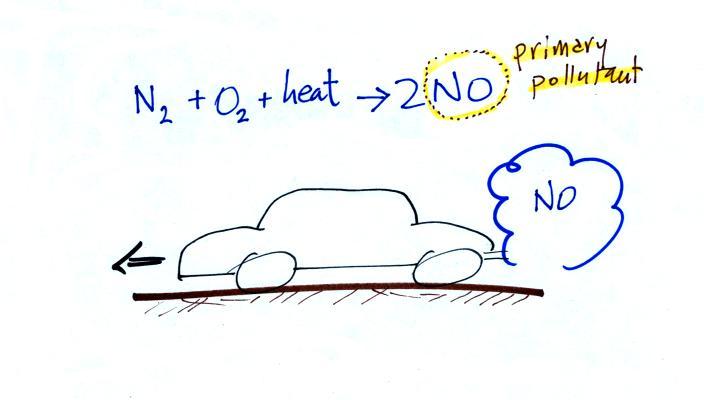
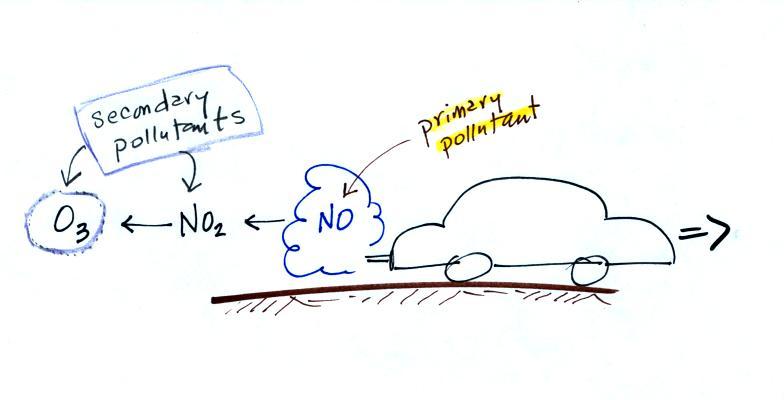
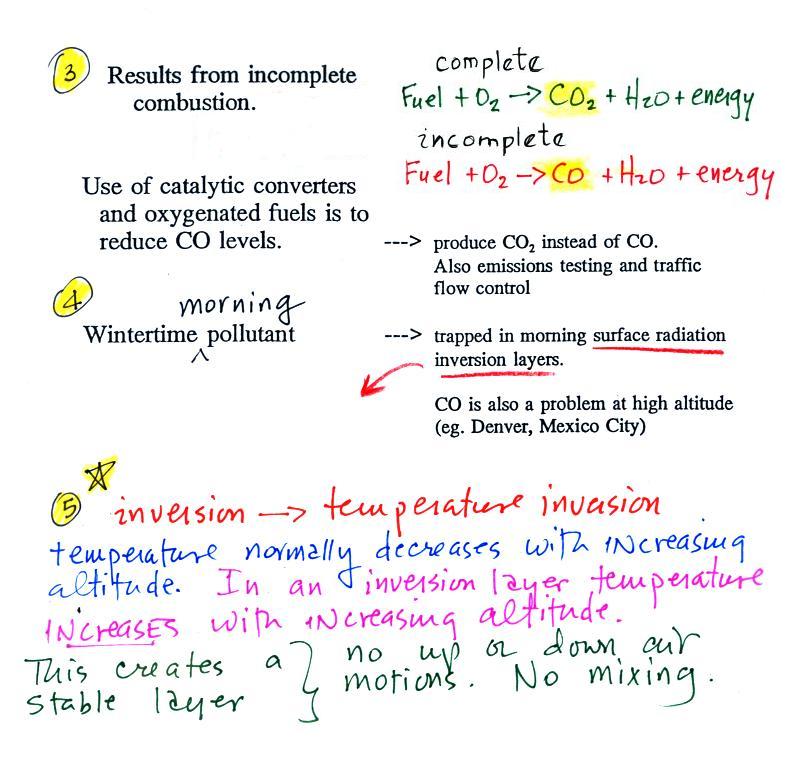
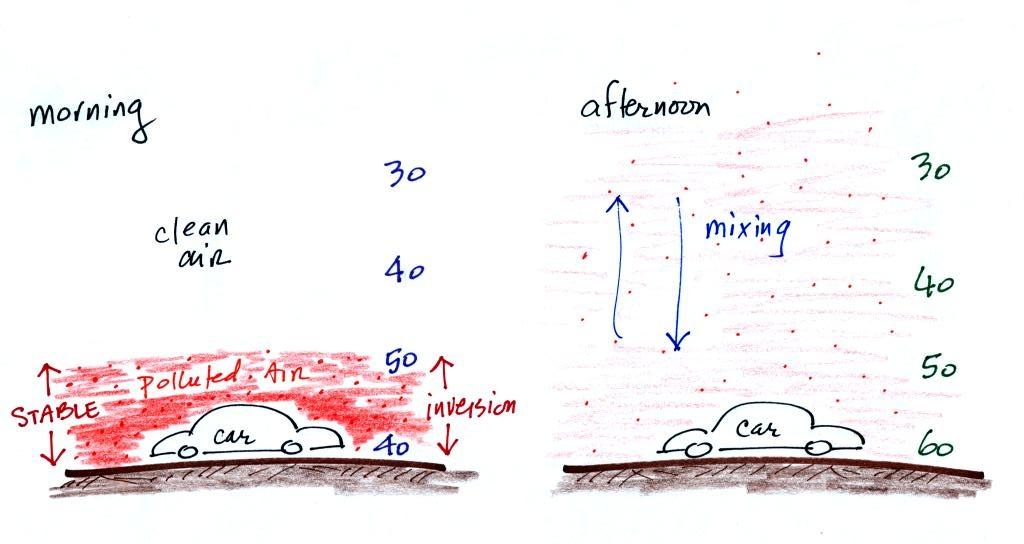
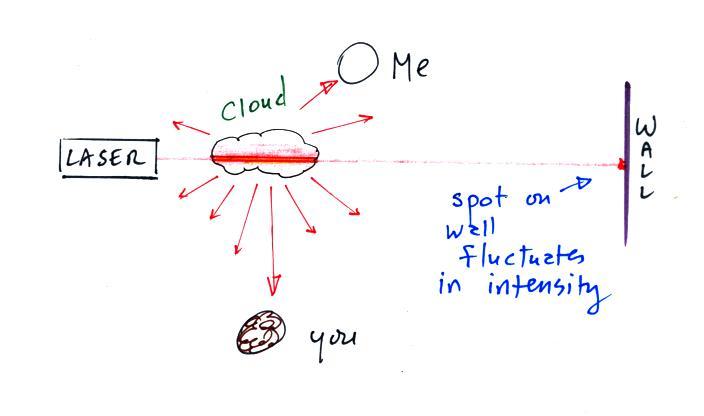
The beam of laser
light really lit up as it passed through the small patches of
cloud. The cloud droplets did a very good job of scattering laser
light. So
much light was scattered
that the spot on the wall fluctuated in intensity (the spot dimmed when
lots of
light was being scattered, and brightened when not as much light was
scattered). Here's a photo I took back in my office.
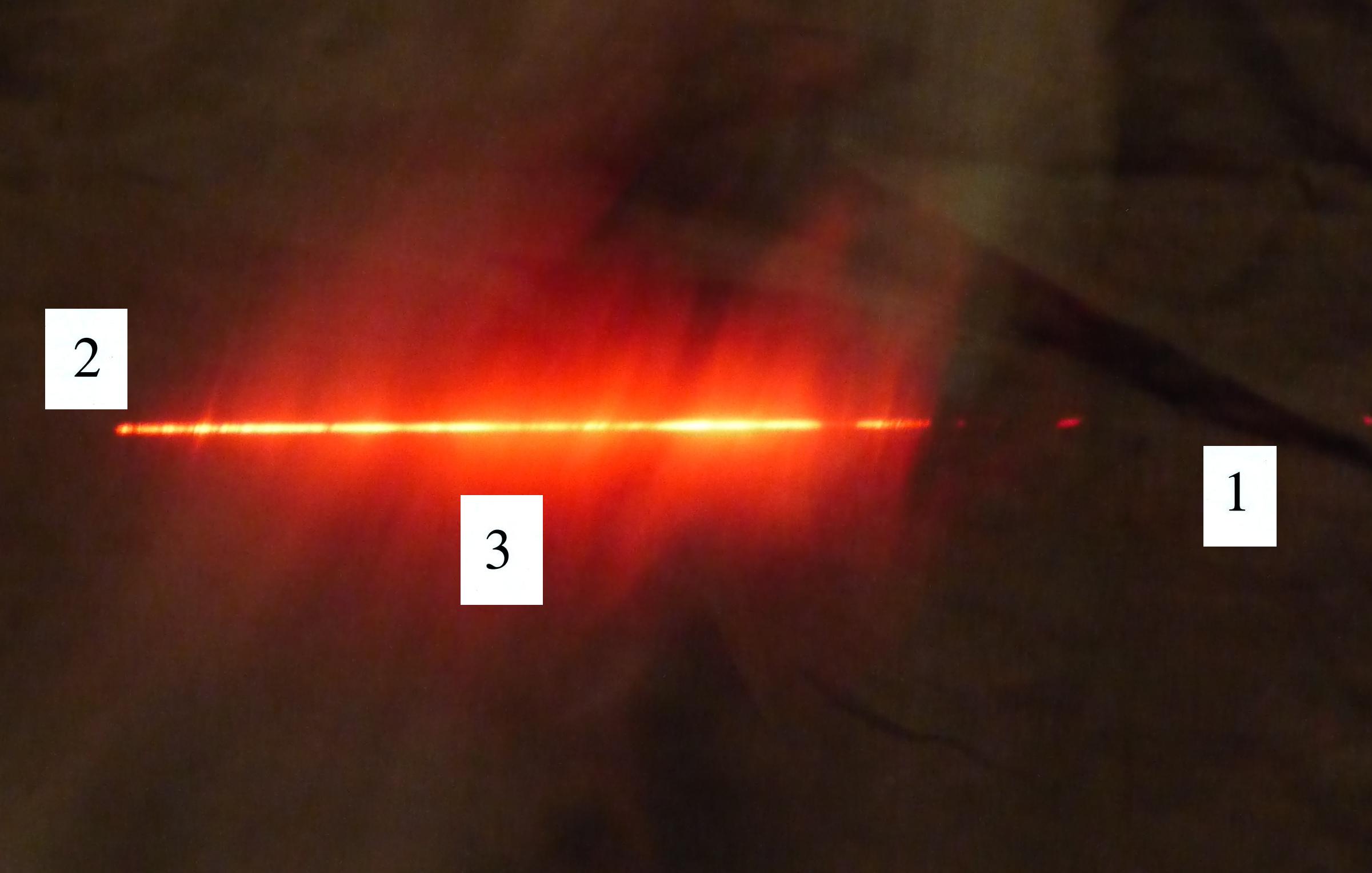
The laser beam is visible in the
left 2/3 rds of the picture
because it is passing through cloud and light is being scattered toward
the camera. There wasn't any cloud on the right 1/3rd of the
picture so you can't see the laser beam over near Point 1.
There's something else going on in this picture also. We're
not just seeing the narrow beam of laser light but some of the cloud
outside the laser beam is also visible.
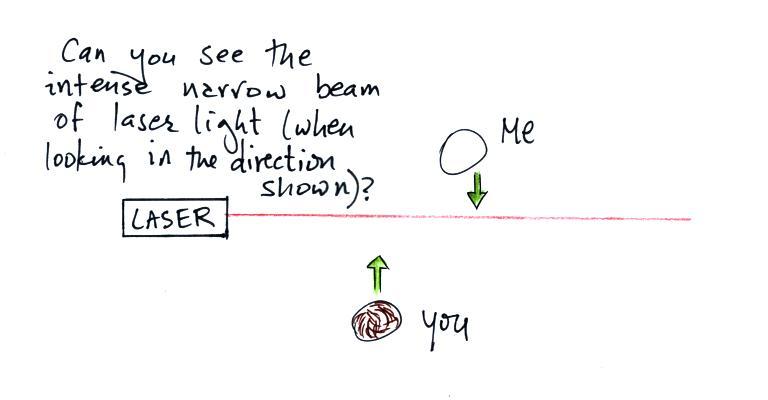
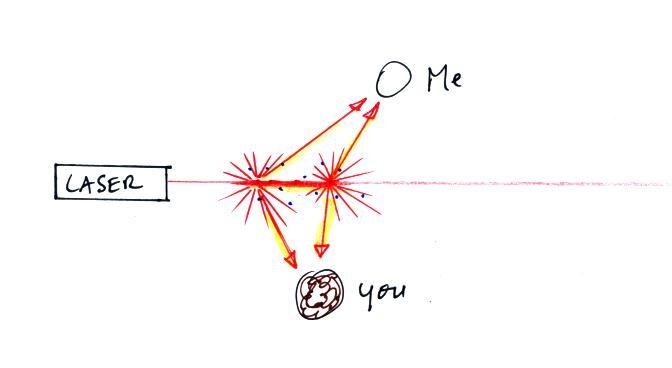
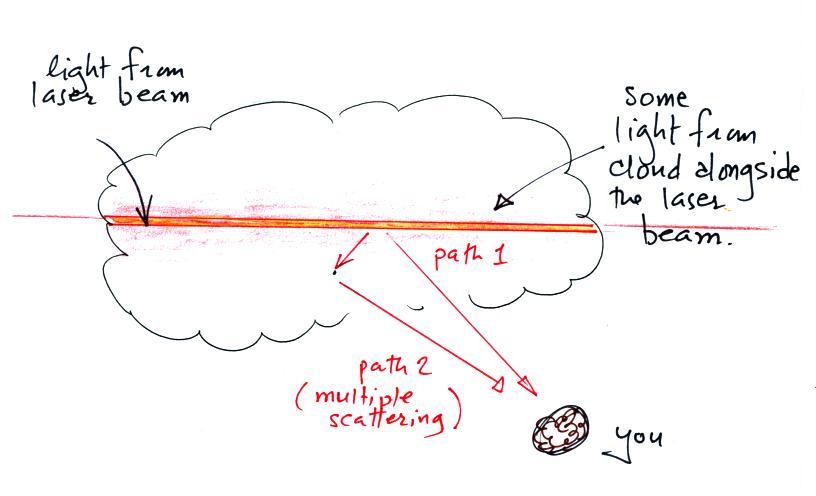
Up to this point we've just considered single scattering. A
beam
of light encounters a cloud droplet or a particle of chalk and gets
redirected and then travels all the way to your eye or to a
camera. That's what's happening at Point 2. You just see
the narrow laser beam. But sometimes the scattered ray of light
runs into
something else and gets scattered again. This is called multiple
scattering. And that is what is illuminating the cloud alongside
the beam of laser light at Point 3. Light is first scattered by a
cloud droplet in the beam. As it leaves the beam it runs into
another droplet and gets scattered again. So now it looks like it
is coming from the cloud surrounding the laser beam rather than from
the beam itself.
Sunlight is
white light which means it's made up of a mixture of violet, blue,
green, yellow, orange, and red
light. Air molecules have an unusual property: they scatter the
shorter wavelengths (violet, blue, green) much more readily than the
longer wavelength colors in sunlight (yellow, orange, and red).
When you look away from the sun and look at the sky, the blue color
that you see are the shorter wavelengths in sunlight that are being
scattered by air molecules.
You shouldn't look directly at the sun. Direct sunlight is
too intense just as was true with the laser. But it is OK to look
at the blue sky. That's scattered sunlight and is much weaker
than direct sunlight and safe to look at.
We'll come back to
this concept of scattering of light in the next couple of lectures.