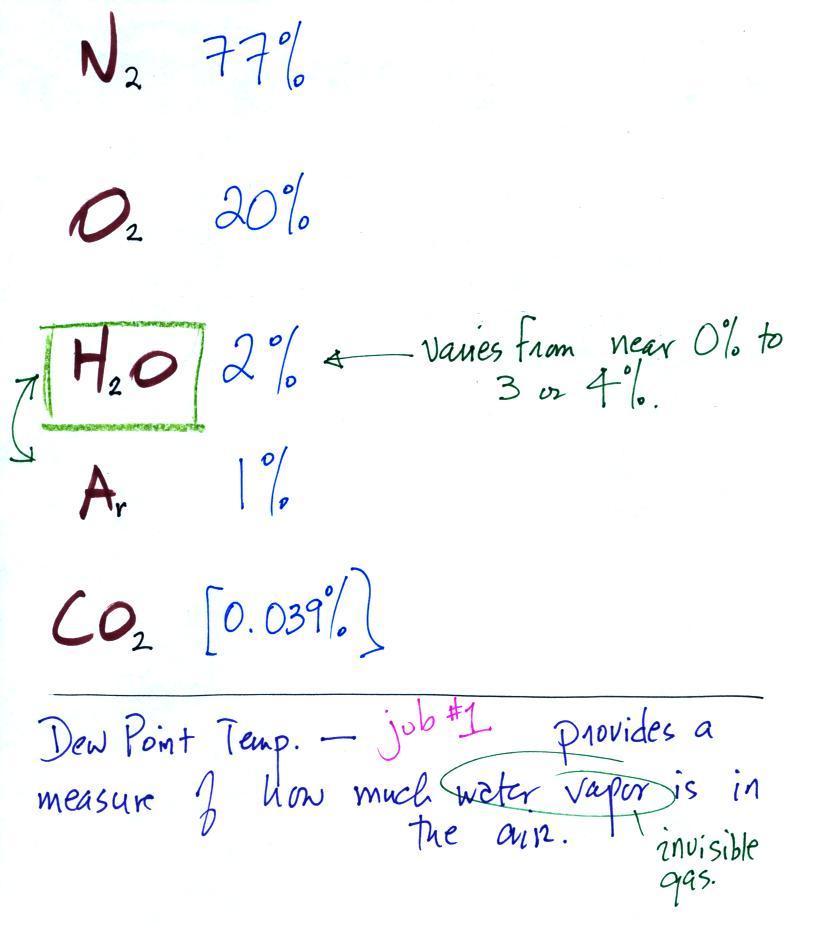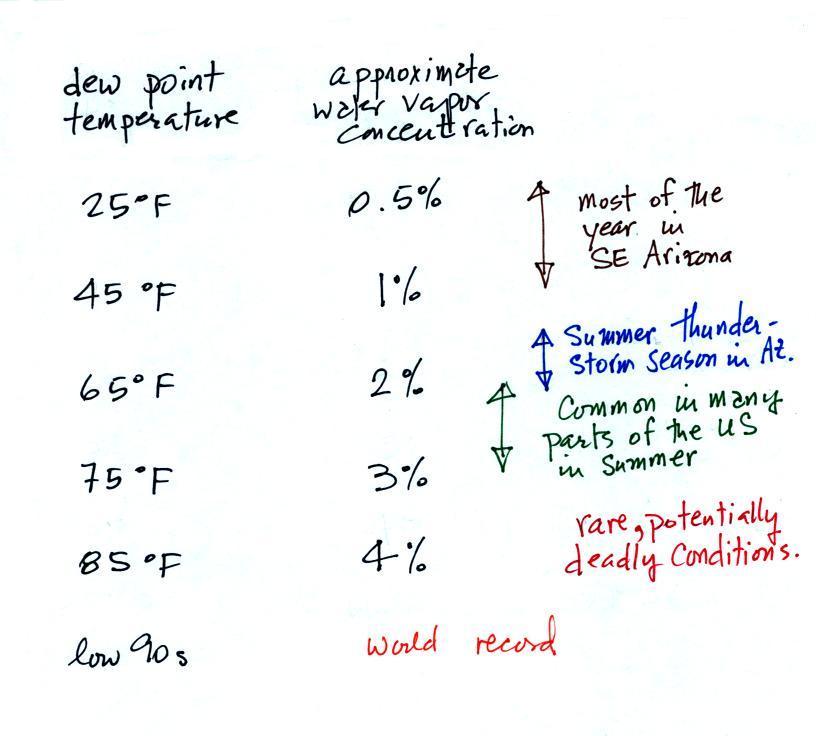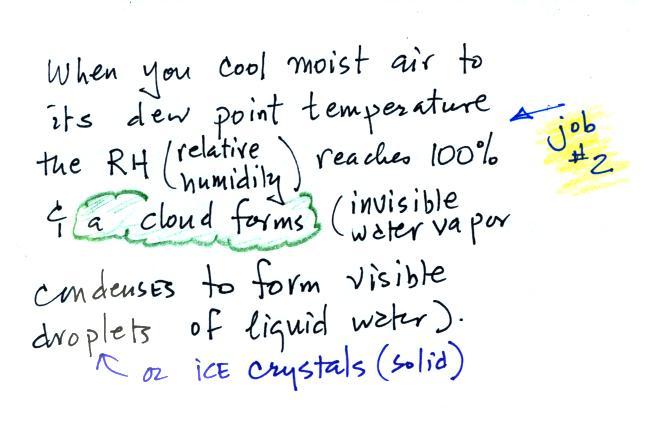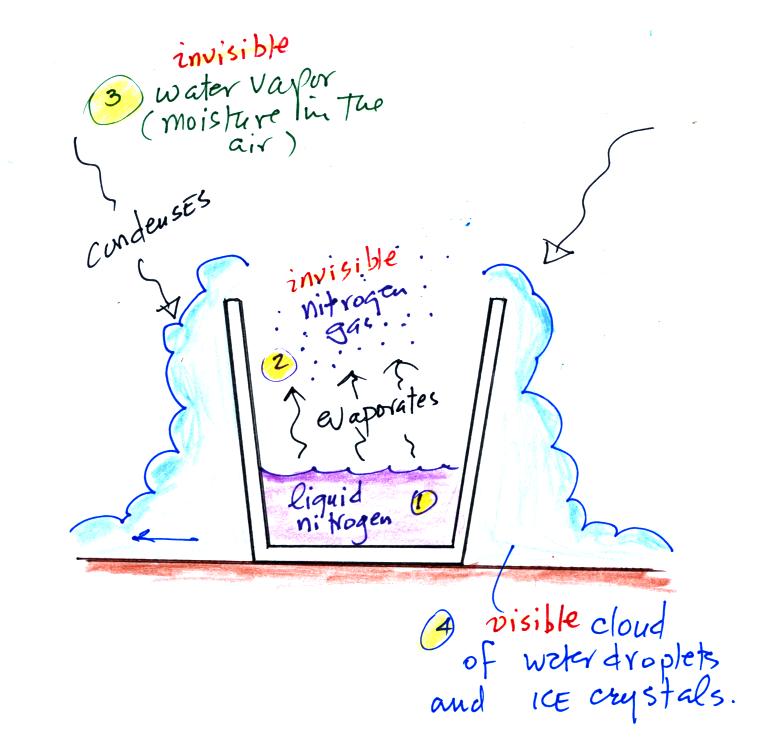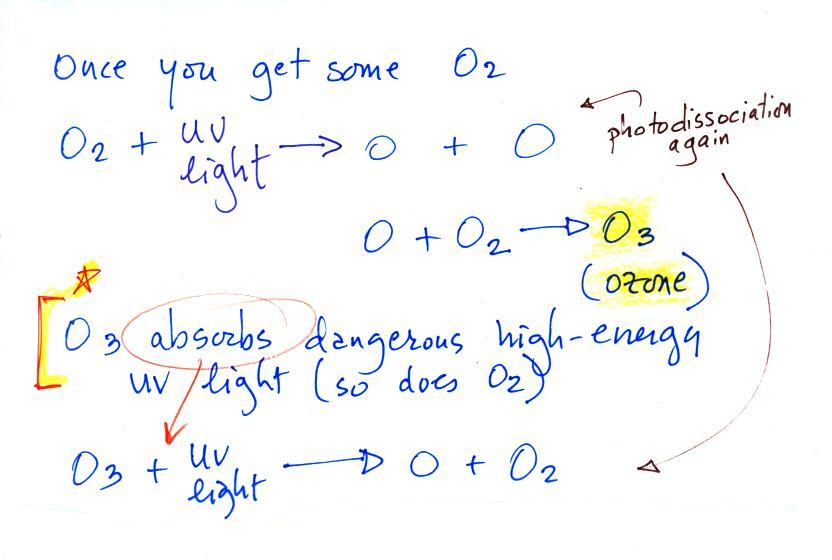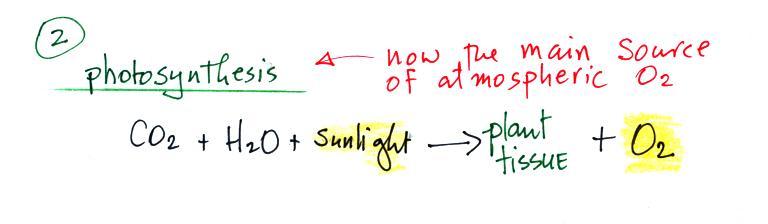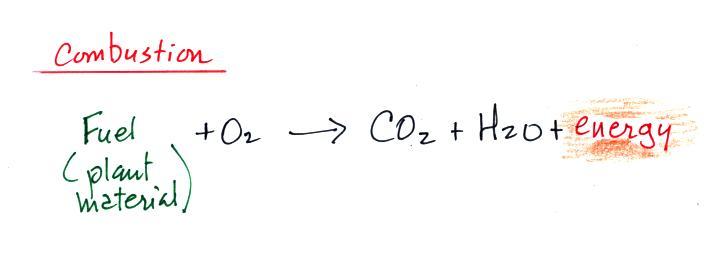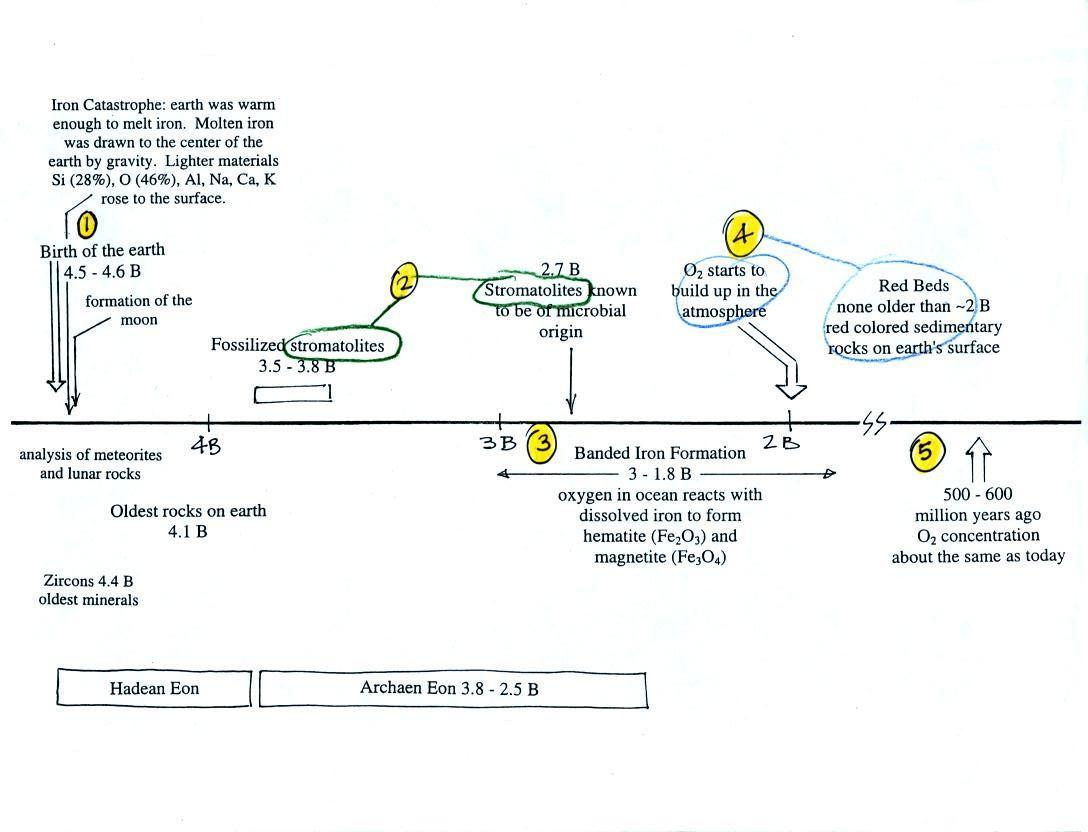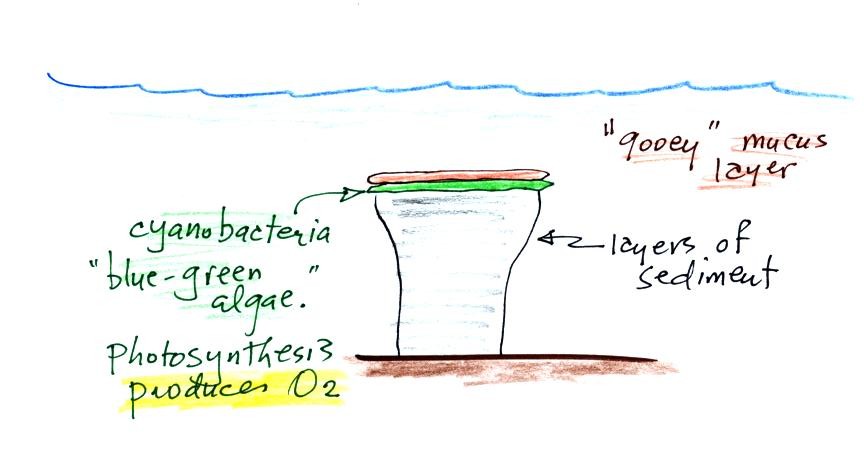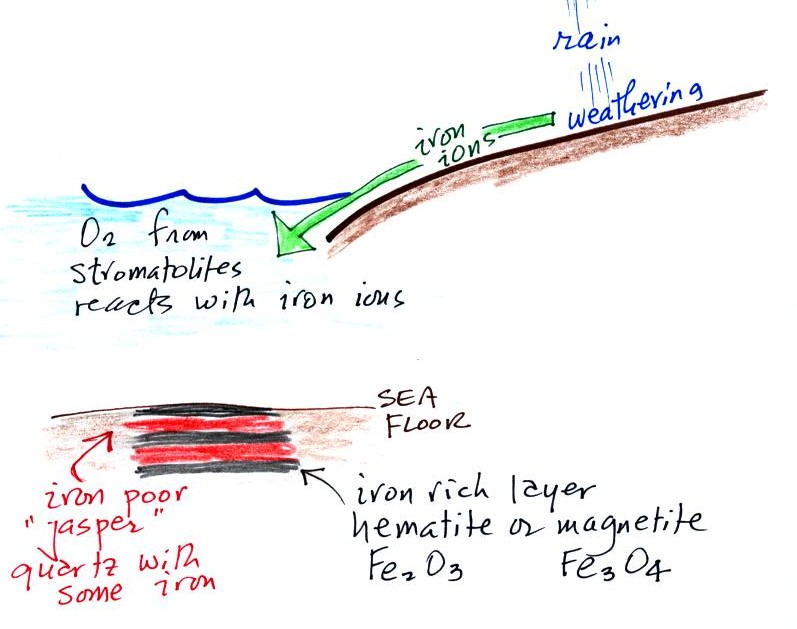
Periodically the oxygen production
would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
in incoming sunlight might have slowed the photosynthesis).
During these times of low
dissolved oxygen concentrations, layers of jasper would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded iron formation. In addition to the
red
and black layers, you see yellow layers made of fibers of quartz in the
samples passed around class. The
rocks are fairly heavy because they contain a lot of iron, but the most
impressive thing about them in my opinion is
their age - they are a few billion years old! And thanks for
returning them by the way.
We were out of time at this point. Rushing through the last
little bit of material in the final minutes of class will be a common
event this semester. I usually try to clear things up in the
online notes.
Eventually the oxygen in the ocean reacted with all of the iron
ions and was free to move from
the ocean into the
atmosphere. Once in the air, the oxygen could react with iron in
sediments on the earth's surface. This produced red colored
(rust colored) sedimentary rock. These are called "Red Beds" (Point 4). None of these
so-called red beds
are older than
about 2 B years old. Thus it appears that a real buildup up
of oxygen in the atmosphere began around 2 B years ago. Oxygen
concentrations reached levels
that are about the same as today around 500 to 600 million years ago (Point 5
in the figure).