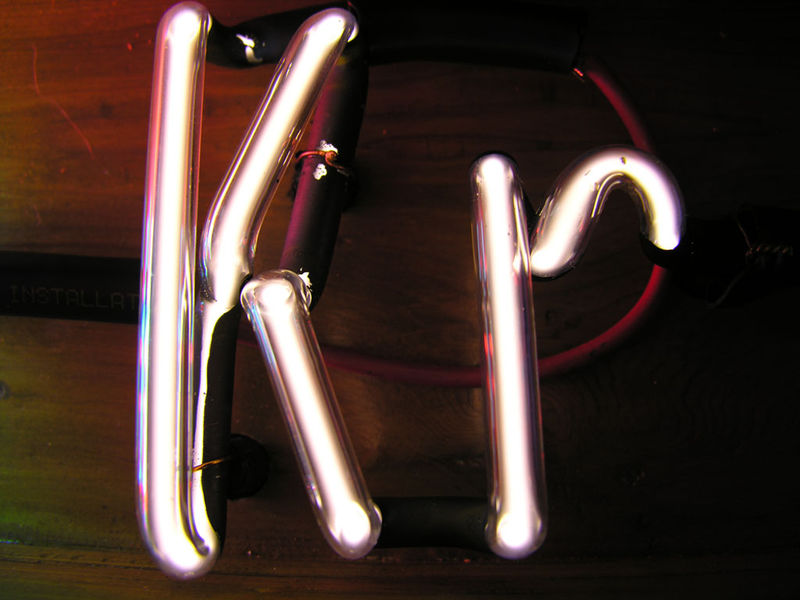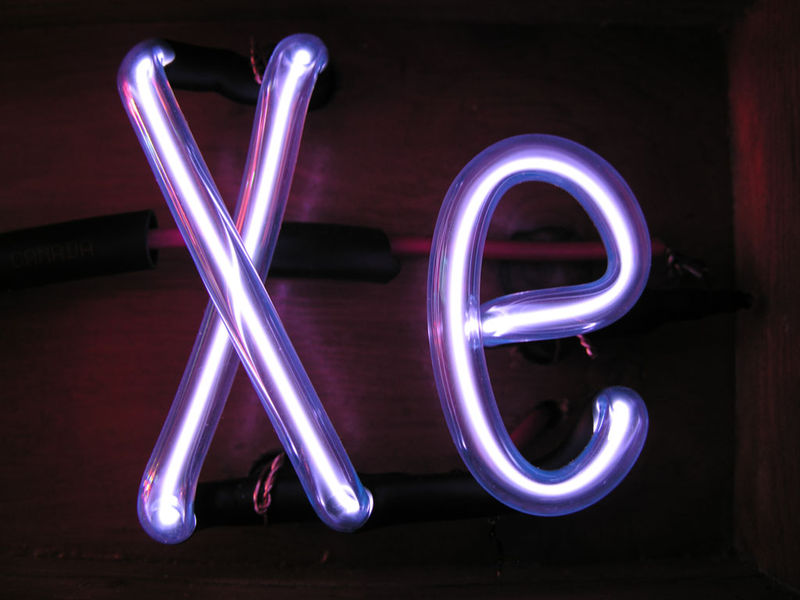Thu., Jan. 15, 2015
Music: Wilsen "House on a
Hill"; Laura Marling "Blackberry
Stone"; Lucius "Turn It
Around"; The Bangles "Hazy Shade of Winter" the 1987 version
is what you heard in class but the Live version
recorded in 2000 is also good. There was time for "Hello, I Love
You" from The Doors before the 9:30 class.
I hope you had a chance to see the fog on Wednesday morning.
Fog is pretty rare in Tucson; a once every 2 years or so
phenomenon. Fog like we had yesterday forms when moist
air at ground level cools to the dew point temperature (dew point
temperature was introduced in today's class). The air
yesterday was moister than normal because it had rained a little
bit the night before.
 |

|
Looking east from my
house in central Tucson Wednesday morning
|
Looking west.
Visibility was about 1/4 mile.
|
Course Introduction
Welcome to the Spring 2015 edition of ATMO 170 Introduction to
Weather & Climate. We first briefly discussed the Course Information handout.
Please read through
that information carefully on your own and let me know if you
have any questions.
A textbook is not required for this class. If you want to
get a more complete picture of the subject than we will have time
to do in class, you might want to purchase one of the textbooks
that are being used in the other ATMO 170A1 sections. Or if
you'd like to borrow one of the copies
of introductory level textbooks that I have in my office,
just let me know. Otherwise you should be able to do
perfectly well in the class by reading the online notes. It
is important to read through the online notes even if you are in
class.
A set of photocopied ClassNotes (available in the ASUA
Bookstore in the Student Union) is required. You should try
to purchase a copy as soon as you can because we will probably be
using the first page in class next Tuesday. You might
know someone with notes from last Fall's class; they'll work fine
this semester also.
Writing is an important part of
this class and is described in more detail on the Writing Requirements handout.
Please have a careful look
at that also and
let me know if you have any questions.
The first half of your writing grade is an experiment
report. You only need to do one of the experiments, so think
about which of the experiments (listed on the handout) you might
like to do. I'll bring signup sheets to class next
Tuesday. I'm also planning on bringing about 40 - 45 sets of
materials next Tuesday. Materials checkout is first come
first served.
The so-called One Side of One Page (1S1P) reports make up the
second part of your writing grade. Topics will appear
periodically during the semester on the class webpage. As
you write reports you will earn points (the exact number of points
will depend on the topic and the quality of your report).
Your goal should be to earn 45 1S1P pts, the maximum number
allowed, by the end of the semester.
You'll be allowed to revise and raise your grade on the first
draft of your experiment report. So you should be able to
earn a pretty high score on that. And, unless you
procrastinate, you can just keep on writing 1S1P reports until
you've earned 45 points. There's no good reason not to earn
a high writing grade.
Grade example
Your final grade in this class will depend on your quiz scores,
how much extra credit you earn (from optional take home and in
class assignments), your writing grade, and (perhaps) your score
on the final exam. A sample grade report from the Fall 2014
class is shown below (the numbers are class averages).
Doe_J
quiz1 -43 (155 pts possible) 72.3% quiz scores
quiz2 -50 (170 pts possible) 70.6%
quiz3 -51 (185 pts possible) 74.4%
quiz4 -45 (175 pts possible) 74.3%
1.5 EC points extra
credit earned on optional assignments
writing scores
writing scores: 34.0 (expt/book report) + 45.0 (1S1P pts)
writing grade: 98.8%
overall averages (prior to the Final
Exam)
average (no quiz scores dropped): 78.1% + 1.5 =
79.6%
average (lowest quiz score dropped): 80.0% + 1.5 = 81.5%
you DO need to take the final exam
27.0 pts missed on the final exam = 73.0%
exam score
overall average is 79.8%
final grade
The 4 quiz grades are shown at the top.
The next entry shows that the average student earned 1.5
points of extra credit points. You will have the opportunity
to earn at least 3 extra credit points.
A score of 34 points on the experiment report and 45 1S1P pts
resulted in a writing percentage grade of 98.8%. There's no
good reason not to end up with a writing score close to 100% (over
even greater than 100%)
The overall average without any quiz scores dropped is shown
next. Since the result, 79.6%, is less than 90.0% the
average student last fall did have to take the final exam
The second average (with the lowest score dropped) is a little
higher, 81.5%.
If you do well on the final exam it will count 40% of
your overall grade (trying to maximize the benefit it can
have). If you don't do so well on the final it only counts
20% (minimizing the damage it can cause). In this example
the final exam score (73 %) was lower than the 81.5% value, so the
final exam only counted 20% and the overall score was 79.8%.
This would be rounded up to 80% and a B.
Note that even though this average student had C grades on all 4
quizzes and the Final Exam, the student ended up with a B in the
class. That is due largely to the high writing grade and the
fact that the student did have some extra credit points.
Comments
about the class
Next a
couple of comments about the class from Spring 2014:
comment #1
The important thing is to keep up with material as
it's covered in class. You don't necessarily need to
come to class to do this. You should definitely be
reading the online lecture notes on a regular basis.
comment #2
Don't let concerns like this wait until the
end of the semester. Let me or one of the TAs know of
your concerns so that they can be addressed during the
semester.
"Chapter 1" - the
earth's atmosphere
We did cover a little course material in class today just so
you can get an idea of how that will work. If we were using a book we'd start
in Chapter 1 and here's some of what we would first be looking
at in this course.
We
will come back to the first item - the composition of
the atmosphere.
Before we do that
however, here are a few questions to get you thinking
about the air around you. This is an example of
material that appears in the online notes even though it
wasn't covered in class. I
usually insert some red bold text to get your
attention.
Example of material appearing in the online notes
even though it wasn't covered in class.
Can
you see air?
Air
is
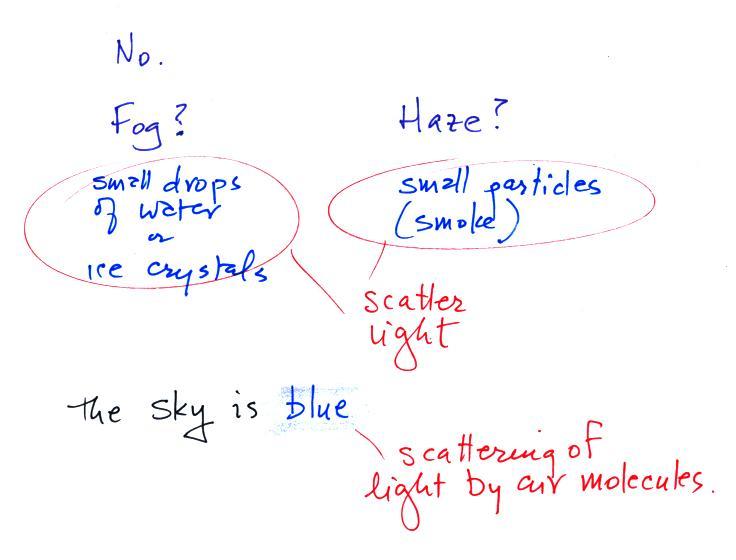
mostly clear, transparent, and invisible (that would be true
of the air in the classroom). Sometimes the air looks
foggy, hazy, or smoggy. In these cases you are "seeing"
small water droplets or ice crystals (fog) or small particles
of dust or smoke (haze and smog). The particles
themselves may be too small to be seen with the naked eye but
are visible because they scatter (redirect) light.
I didn't really mention or explain what that is but it's a pretty
important concept and we will learn more about it in a
week or so.
And to be completely honest air isn't really invisible.
If you shine a bright light through enough air, such as when
sunlight shines through the atmosphere, the air (the sky)
appears blue. This is a little more complicated form of
scattering of sunlight by air molecules. We'll come back
to this later as well.
Can you
smell air?
I don't think you can smell or
taste air (air containing nitrogen, oxygen, water vapor, argon
and carbon dioxide). But there are also lots of other odors
you can sometimes smell (freshly cut grass, hamburgers on a
grill, etc). I don't consider these normal constituents of
the atmosphere.
You can probably also smell certain pollutants. I suspect
our sense of smell is sensitive enough for us to detect certain
air pollutants even when their concentration is very small
(probably a good thing because many of them are
poisonous).
Natural gas (methane) used in
hot water heaters, some stoves, and furnaces is odorless.
A chemical (mercaptan) is added to natural gas so that you can
smell it and know when there is a leak before it builds up to a
concentration that could cause an explosion.
Can you feel air
It is harder to answer this question.
We're always in contact with air. Maybe we've grown so
accustomed to it we aren't aware of how it feels. We can
certainly feel whether the air is hot or cold, but that have
more to do with energy exchange between us and our
surroundings. And we can feel wind.
In a week or two we will see that, here in the classroom, air
pressure is pressing on every square inch of our bodies with 12
or 13 pounds of force. If that were to change suddenly I'm
pretty sure we'd feel it and it would probably really hurt.
What are the 5 most abundant
gases in air?
Let's start with the most abundant gas in the atmosphere.
I poured some of this same material (in liquid form) into a
Styrofoam cup. Here's a photo I took back in my office.
You can see the liquid, it's
clear, it looks like water. I had the impression
that a lot of students knew this was liquid
nitrogen. It's very cold and begins to boil
(evaporate) at -321o
F.
The most abundant gas in the atmosphere is nitrogen.
We'll use liquid nitrogen in several class demonstration this
semester mostly because it is so cold.
Nitrogen was discovered in 1772 by Daniel Rutherford (a
Scottish botanist). Atmospheric nitrogen is relatively
unreactive and is sometimes used to replace air in packaged
foods to preserve freshness. You don't need to worry
about details like this for a quiz.
Oxygen is the second most abundant gas in the
atmosphere. Oxygen is the most abundant element (by
mass) in the earth's crust, in ocean water, and in the human
body.
A couple of photographs of
liquid oxygen are shown above (it
boils at -297o F).
It has a (very faint) pale blue
color (I was pretty disappointed because I had
imagined it might be a deep vivid blue). I'd
love to bring some liquid oxygen to class but I'm not
sure it's available on campus. Also oxygen is
very reactive and I suspect you'd need to be very
careful with liquid oxygen.
When
heated (such as in an automobile engine) the oxygen and
nitrogen in air react to form compounds such as nitric
oxide (NO), nitrogen dioxide (NO2), and nitrous
oxide (N2O). Together as a group these
are called oxides of nitrogen; the first two are air
pollutants, the last is a greenhouse gas.
Here is a complete list of the 5 most abundant gases in
air. And a note about the
figures you'll find in these online notes.
They may differ somewhat from what was done in class. I
often redraw them after class, or use neater versions from a
previous semester for improved clarity (and so I can get the
notes online more quickly).

Water vapor and argon are the 3rd and 4th most abundant gases
in the atmosphere. A 2% water vapor concentration is listed
above but it can vary from near 0% to as high as 3% or 4%.
Water vapor is, in many locations, the 3rd most abundant gas in
air. In Tucson most of the year, the air is dry enough that
argon is in 3rd position and water vapor is 4th.
Water vapor, a gas, is invisible. Water is the only
compound that exists naturally in solid, liquid, and gaseous
phases in the atmosphere.
Argon is an unreactive noble gas (helium, neon, krypton, xenon, and radon are also inert gases).
Here's a picture of solid argon (argon
"ice"). It melts at melts at -309o
F and evaporates
at -302o F; it's doing both in this
picture. (image
source).
This is solid carbon dioxide, better known as
dry ice. It doesn't melt, it sublimates. I.e. it
changes directly from solid to gas. (
source)
The concentration of carbon dioxide is much smaller than the
other gases (you don't need to remember the actual value).
That doesn't mean it isn't important. We'll spend a lot of
time this semester talking about water vapor and also carbon
dioxide. Water vapor and carbon dioxide are the two best
known and most important greenhouse gases. The greenhouse
effect warms the earth. Concentrations of greenhouse gases
such as carbon dioxide are increasing and there is concern this
will strengthen the greenhouse effect and cause global
warming. That's a topic we'll look at during the semester.
Here's a little more
explanation (from Wikipedia)
of why noble gases are so unreactive. Don't worry about
all these additional details, none of
this was covered in class. The noble gases
have full valence
electron shells. Valence electrons are the outermost electrons of an atom and are normally the only
electrons that participate in chemical bonding. Atoms with full valence
electron shells are extremely stable and therefore do not tend
to form chemical bonds and have little tendency to gain or lose
electrons (take electrons from or give electrons to atoms of
different materials).
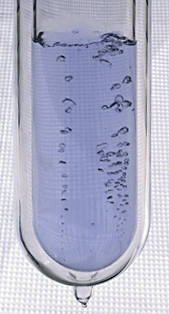
Noble gases are often used used in neon signs; argon produces a
blue color. The colors produced by Argon (Ar), Helium (He),
Kryton (Kr), Neon (Ne) and Xenon (Xe), which are also noble gases,
are shown above (source of the
images). The inert gases don't react with
the metal electrodes in the bulbs. Neon bulbs and
fluorescent bulbs (including energy saving CFLs) often also
contain mercury vapor (which means you should dispose of them
carefully when they burn out). The mercury vapor emits
ultraviolet light that strikes phosphors of different kinds on the
inside of the bulb. Different colors are emitted depending
on the particular type of phosphor used in the bulb.
If we were using a textbook we'd probably find something like
the following table near the beginning of the book ( I found this
table a few years ago in a Wikipedia
article about the earth's atmosphere ).
I like our list of the 5 most abundant gases better. It's
much more manageable. There is almost too much information
in a chart like this, you might be overwhelmed and not remember
much. Also unless you are familiar with the units on the
numbers they might be confusing. And notice you don't find
water vapor in 3rd or 4th position near the top of the
chart. That's because this is a list of the gases in dry
air. Unless you're very attentive, you might miss that fact
and might not see water vapor way down at the bottom of the chart.
If you click on the link above to the Wikipedia article on the
earth's atmosphere you'll find that the list above has been
replaced with a shorter simpler list (much more like the one we
created in class).
Dew point temperature
Water plays an important role in the formation of
clouds, storms, and weather. Meteorologists are very
interested in knowing and keeping track of how much water vapor is
in the air at a particular place and time. One of the
variables they use is the dew point temperature. The value
of the dew point gives you an idea of how much water vapor is
actually in the air. A high dew point value means more water
vapor in the air and higher the water vapor concentration.

The chart below gives a rough equivalence
between dew point temperature and percentage concentration of
water vapor in the air. And
I did mess the table up in class, the correct version is
shown below.

I should have remembered that
water vapor concentration doubles every time the dew point
temperature increases 20o F,
I would have gotten it right.
Air temperature will always be equal to or warmer than the
dew point temperature. Experiencing 80o F dew points would be
very unpleasant and possibly life threatening because your
body might not be able to cool itself ( the air
temperature would probably be in the 90s or maybe even
warmer). You could get
heatstroke and die.
Click here
to see current dew point temperatures across the U.S. Here's
a
link concerning unusually high, even record setting dew
point temperatures.
Some additional information not
mentioned in class. At one time the dew
point temperature was used to identify the official start of
the summer monsoon season in Tucson (the summer thunderstorm
season). A
monsoon is a seasonal change in
the direction of the prevailing winds. Most of
the year in Tucson winds come from the west and are dry.
For 2 or 3 months in the summer the winds turn and blow from
the east or southeast and the air is much moister.
During most of the year the dew point will fall between 25o F and 45o F. Dew points
in the summer usually range between 55o F and 65o F or
70o F.
Traditionally the summer monsoon would start when the daily
average dew point remained at or above 54 F for three days in
a row.
Dew point temperature continued
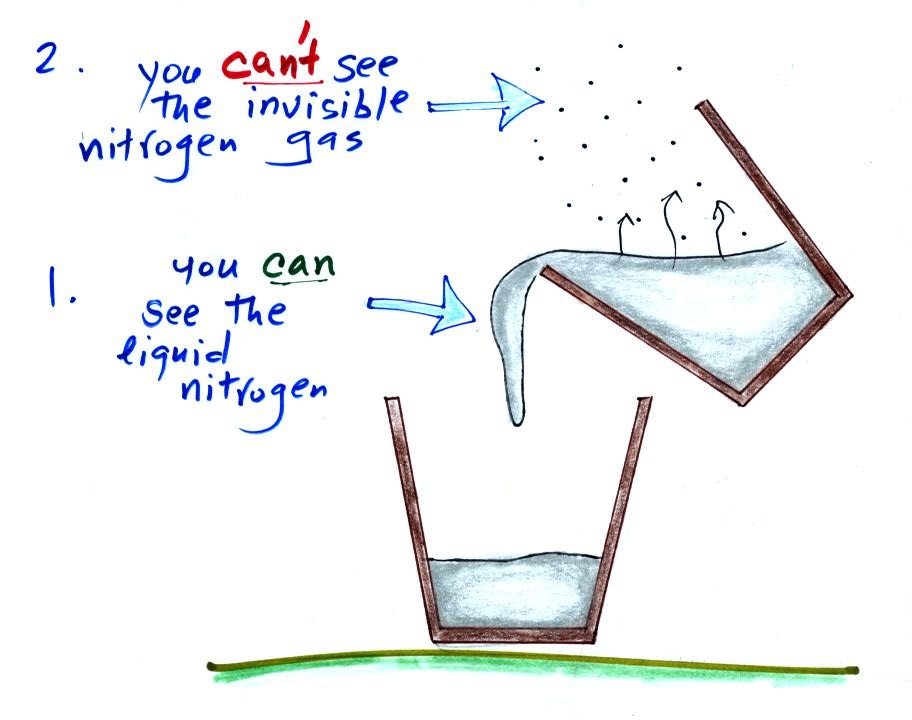
Now let's go back to the cup
of liquid nitrogen
We can see liquid
nitrogen but we can't see the nitrogen gas being
produced by the evaporation of liquid nitrogen. The
white cloud that surrounds the cup of liquid nitrogen
isn't nitrogen gas, what is it? There was also some
frost or snow on the side of the cup, it and the cloud are
made of the same material.
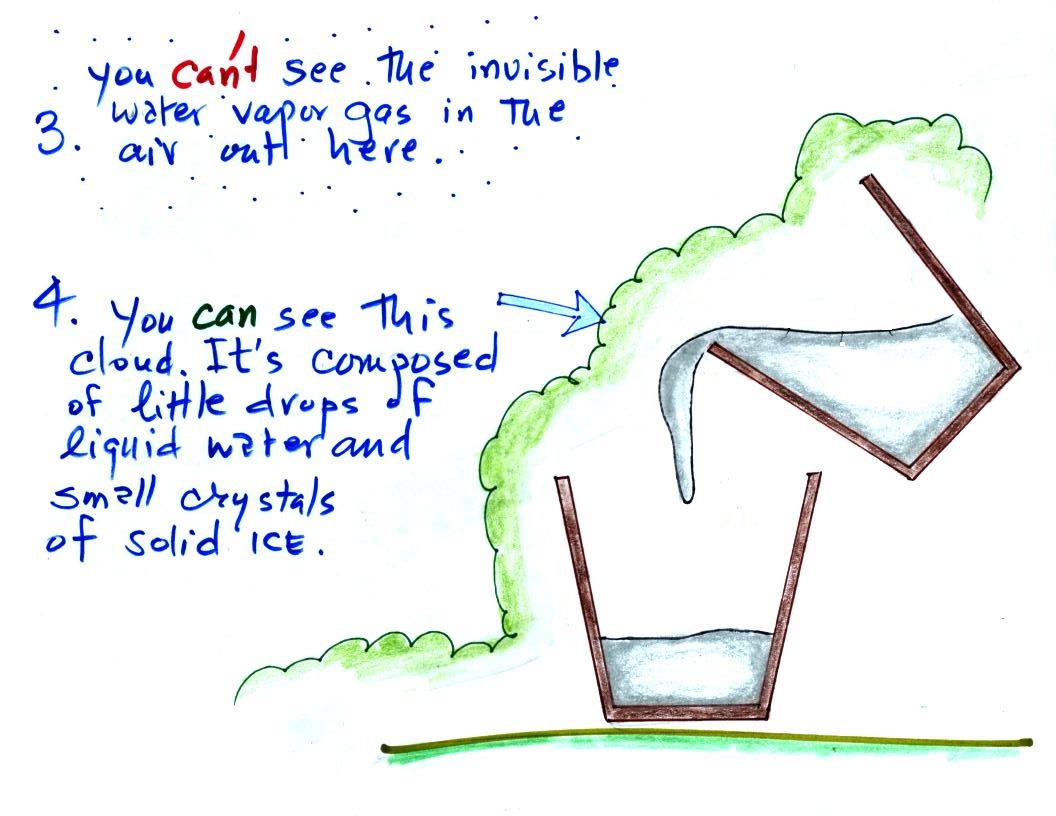
The white cloud isn't water vapor because water vapor, a gas,
is invisible just like nitrogen gas. When the air is cooled
however, by coming into contact with the liquid nitrogen, the
water vapor condenses and forms small droplets of water (liquid)
or ice crystals (solid). That's what you are
able to see, a cloud composed of water droplets or ice crystals.
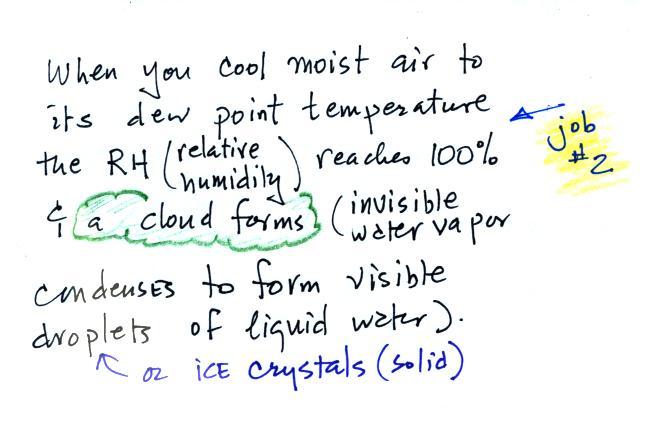
We're seeing a demonstration of the dew point's second job.
If you cool air next to the ground to its dew point, water
vapor will condense and coat the ground with water. The
ground will be covered with dew. If a little thicker layer
of air is cooled fog will form.
Here's a
link to an article about Wednesday's fog that appeared in
the Arizona Daily Star. This type of fog is called radiation
fog or valley fog. The fog cloud is often very thin.
You can't see very far if you look horizontally, but you can often
look up through the fog and sometimes see blue sky.
Closing remarks and Pluto's Gate to Hell
We were nearing a full hour at this point which
is probably enough for the first day of class.
When you have a free moment try to recall the 5 most abundant
gases in air without looking at your notes. Then try to
remember something about each of them. You'll find what I
think is a reasonable list at the end of today's notes. Try
to remember the two jobs of the dew point temperature. Doing
this will dramatically increase the odds of remembering this
information a week or two from now.
Pluto's
Gate to Hell was discovered in early
2013 at the ancient city of
Hierapolis in southwestern Turkey
(Pluto was the Roman god of the
underworld, he was called Hades by
the Greeks). None of what
follows was covered in class.
The
picture above at left shows the
site as it appears now (source
of this photograph).
The gate is the opening in the
wall near the center of the
picture. The site as it
might have appear in ancient
times is shown above at
right. This photograph,
credited to Francesco
D'Andria, the lead Italian
archaeologist that announced
the discovery in March 2013,
is found in a news
report from the National
Geographic Society.
The
"gate" was built on top of a
cavern and, in ancient times,
a mist of deadly vapors could
be seen coming from the cave
(the mist is shown in the
right picture above).
Here's a quote from the Slate
article where I first
read about the discovery:
"Two
millennia ago, visitors to
Pluto's Gate could buy small
birds or other animals (the sale
of which supported the temple)
and test out the toxic air that
blew out of the mysterious
cavern. Only the priests,
high and hallucinating on the
fumes, could stand on the steps
by the opening to hell.
They would sometimes lead
sacrificial bulls inside, later
pulling out their dead bodies in
front of an awed crowd.
As
the Greek geographer,
philosopher, and prolific
traveler Strabo, who lived from
64/63 B.C. to 24 A.D., so
enticingly described it: 'This
space is full of a vapor so
misty and dense that one can
scarcely see the ground.
Any animal that passes inside
meets instant death. I
threw in sparrows and they
immediately breathed their last
and fell.' "
The Italian archaeologists working at the
site would occasionally notice birds dying if they flew into
the vapors coming from the came. The deadly gas was
carbon dioxide. Carbon dioxide is not ordinarily thought
of as a poisonous gas but in high enough concentrations it can
asphyxiate you (cause you to suffocate).
Here's what you might be able to recall about the 5
most abundant gases in our atmosphere.