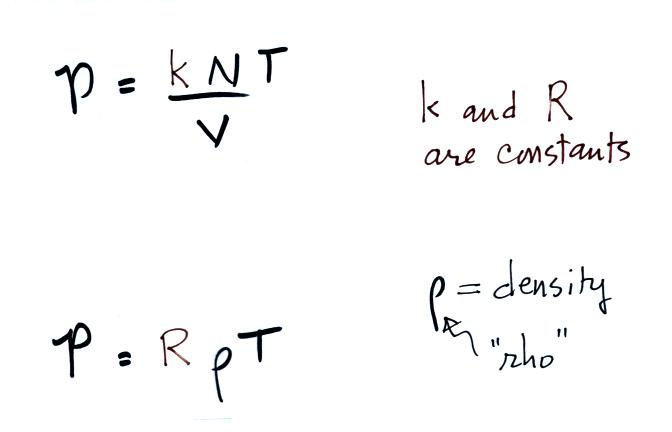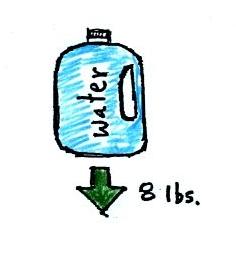Tuesday Feb. 10, 2015
Songs this morning selected from Dave McGraw and
Mandy Fer: "Slumbering
Rose" (5:25), "Grow"
(4:18), "Serotiny"
(4:53), "Comin'
Down" (5:13).
The Practice Quiz has been graded and was returned in class
today.
The Practice Quiz has been graded and was
returned in class today. You'll find the number of
points missed (out of 150 points total) written in the upper
left hand corner on the front side of your quiz. The
average grade was -58 which works out to be 61%, which as
you can see below, is very typical for the Practice Quiz for
the spring semester.
Semester
|
8 am class
|
9:30 am class
|
10 am class
|
2 pm class
|
Semester
|
8 am class
|
9:30 am class
|
2 pm class
|
S15
|
61
|
59
|
|
|
|
|
|
|
S14
|
|
|
63
|
62
|
F14
|
66
|
64
|
|
S13
|
---
|
|
|
72
|
F13
|
63
|
67
|
|
S12
|
63
|
|
|
61
|
F12
|
66
|
|
66
|
S11
|
---
|
|
|
62
|
F11
|
65
|
|
65
|
S10
|
61
|
|
|
62
|
F10
|
67
|
|
60
|
S09
|
---
|
|
|
60
|
F09
|
68
|
|
66
|
S08
|
66
|
|
|
64
|
F08
|
65
|
|
65
|
You should download
the Practice Quiz if you didn't take it just to become
familiar with the format. Some similar questions,
maybe even the same questions, will be on Quiz #1 scheduled
for Feb. 19. Answers to the questions on the Practice
Quiz can be found here.
The Experiment #1 reports were collected today. It
takes some time to get those graded. You can expect
them back sometime next week. I plan to have as many
Expt. #2 sets of materials as I can prepare in class on
Thursday. Checkout will be first come first
served. If you haven't yet returned your Expt. #1
materials please do so as soon as you can, we need the
graduated cylinders for Expt. #2. We're starting to
work on the 1S1P reports. The radon reports should be
graded by Thursday this week.
A take home Optional
Assignment was handed out in class today. It is
due one week from today (Feb. 17). To earn full extra
credit on the assignment it must be done before you come to
class.
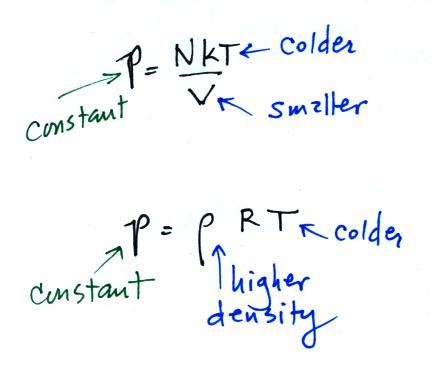
We were trying to understand why warm air rises and cold air
sinks before the Practice Quiz last Thursday. The first
step was learning about the ideal gas law:
We'll be working on the
two remaining steps today.
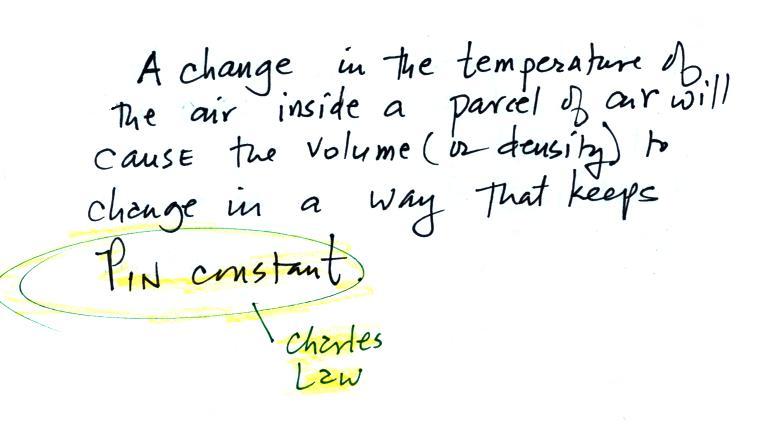
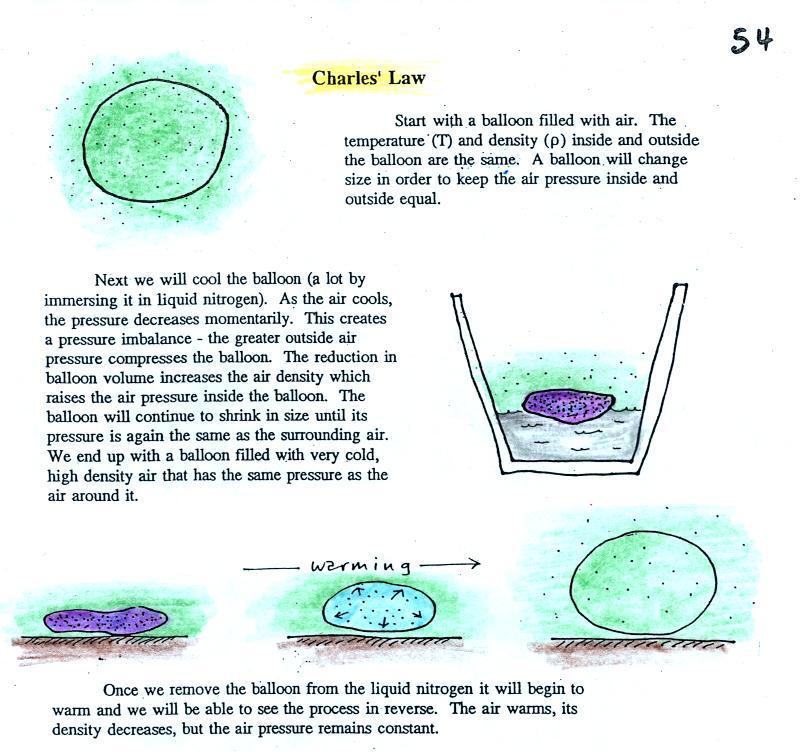
Step #2 Charles' Law
Charles Law requires that the pressure of
a parcel of air remain constant (parcel is just another word
for volume). Changing the temperature of a volume of
air will cause a change in density and volume; pressure will
stay constant. This is an important situation because
this is how volumes of air in the atmosphere behave.
This is probably the most difficult part of today's class
and is worked out in lots of detail.
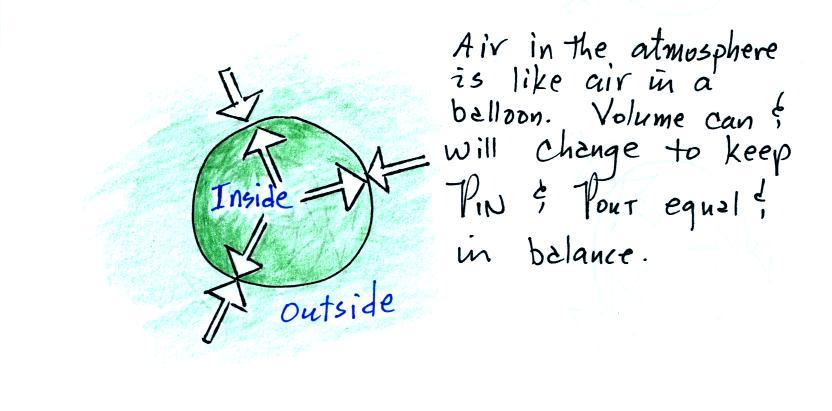
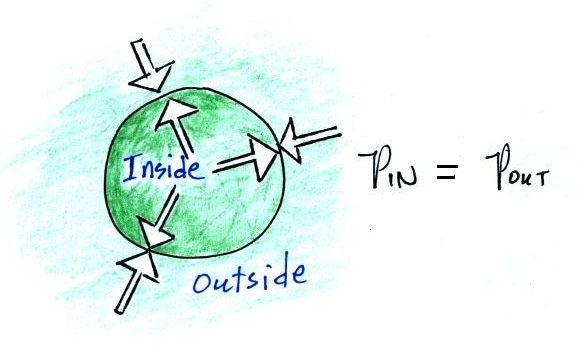
We start with a balloon of air.
The air inside and outside the balloon (or parcel) are
exactly the same.
Note the pressure pushing inward is balanced by the
pressure of the air inside the balloon that is pushing
outward. If we change something inside the balloon
that upsets this pressure balance, the balloon would
expand or shrink until the pressures were again in
balance.
Volumes of air in the atmosphere will always try to keep
the pressure of the air inside the parcel constant (P inside
is always trying to stay equal to P outside). That's
why we say air in the atmosphere obeys Charles' Law.
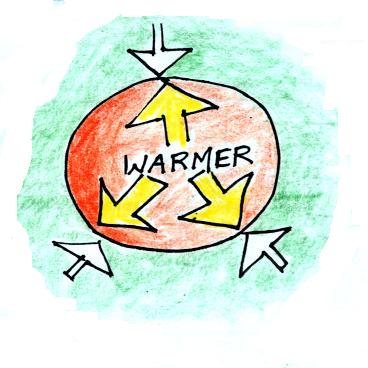
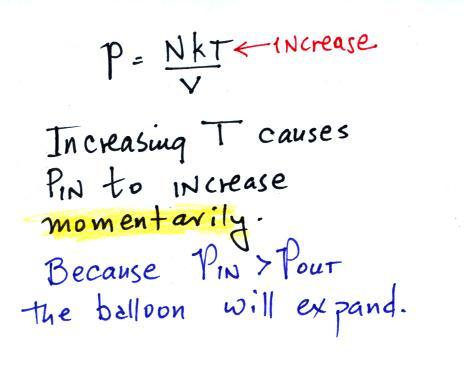
First let's imagine warming the air inside a balloon.
We'll won't change the temperature of the air outside the
balloon.
Increasing the temperature
will BRIEFLY and MOMENTARILY increase the
pressure. This creates an imbalance. Now
that P inside is greater than P outside the balloon will
expand.
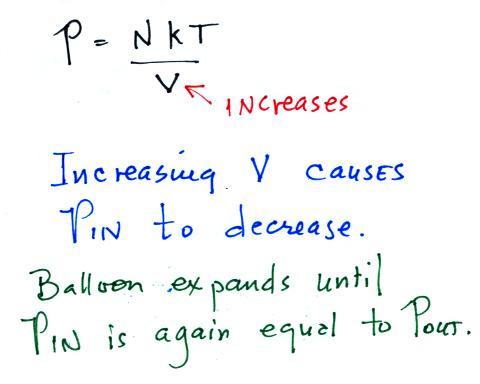
Increasing the volume
causes the pressure to start to decrease. The
balloon will keep expanding until P inside is back in
balance with P outside.
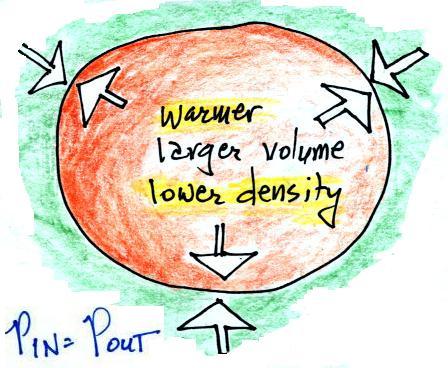
We're left with a balloon that is larger, warmer, and
filled with lower density air than it had
originally.
The pressures inside and outside are again the
same. The pressure inside is back to what it was
before we warmed the air in the balloon. You can
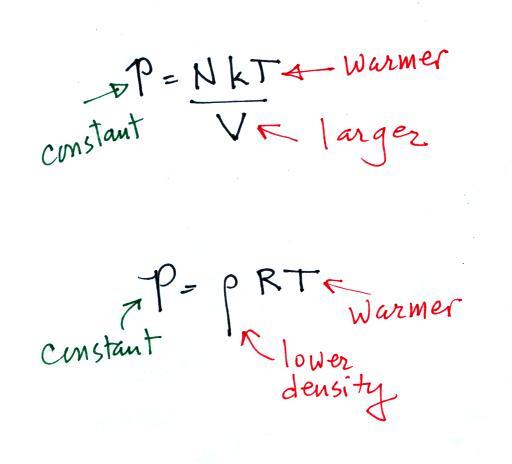
increase the temperature and volume of a parcel together in
a way that keeps pressure constant (which is what Charles'
law requires). Or you can increase the temperature and
decrease the density together and keep the pressure
constant.
In nature the change in temperature and volume occur
simultaneously. It's like jumping from the first to
the last step above.
We can go through the same kind
of reasoning and see what happens if we cool the air
in a parcel. I've included all the steps
below; that wasn't done in
class.
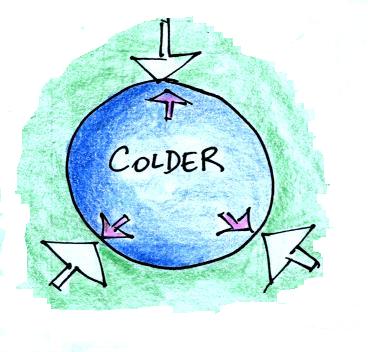
We'll start with a parcel of air that has the same
temperature and density as the air around it.
We'll cool the air inside the parcel. The air
outside stays the same.
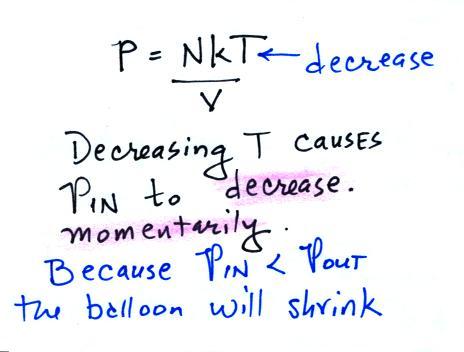
Reducing the air temperature causes the pressure of the
air inside the balloon to decrease. Because the
outside air pressure is greater than the pressure inside the
balloon the parcel is compressed.
The balloon will get smaller and smaller (and the
pressure inside will get bigger and bigger) until the
pressures inside and outside the balloon are again
equal. The pressure inside is back to the value it had
before you cooled the air in the parcel.
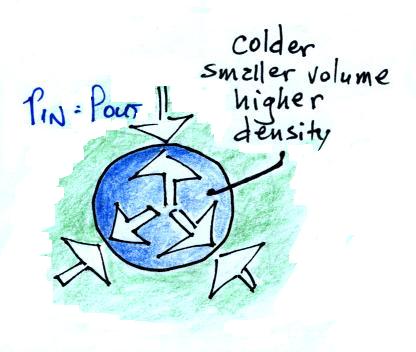
The first and last steps, without all the intermediate
and momentary details, are shown below.
Cooling some air will cause volume to decrease and
density to increase while pressure stays constant.
If you want to skip all the details
and just remember one thing, here's what I'd recommend
Parcels of atmospheric air and air in balloons
behave the same way, they both obey Charles' Law. Charles
Law can be demonstrated by dipping a balloon in liquid
nitrogen. You'll find an explanation on the top of p.
54 in the photocopied ClassNotes.
The balloon shrinks down to practically nothing when
dunked in the liquid nitrogen. It is filled with very
cold, very high density air. When the balloon is
pulled from the liquid nitrogen and starts to warm up it
expands. Density in the balloon decreases. The
volume and temperature keep changing in a way that kept
pressure constant (pressure inside the balloon is staying
equal to the air pressure outside the balloon).
Eventually the balloon ends up back at room temperature
(unless it pops while warming up).
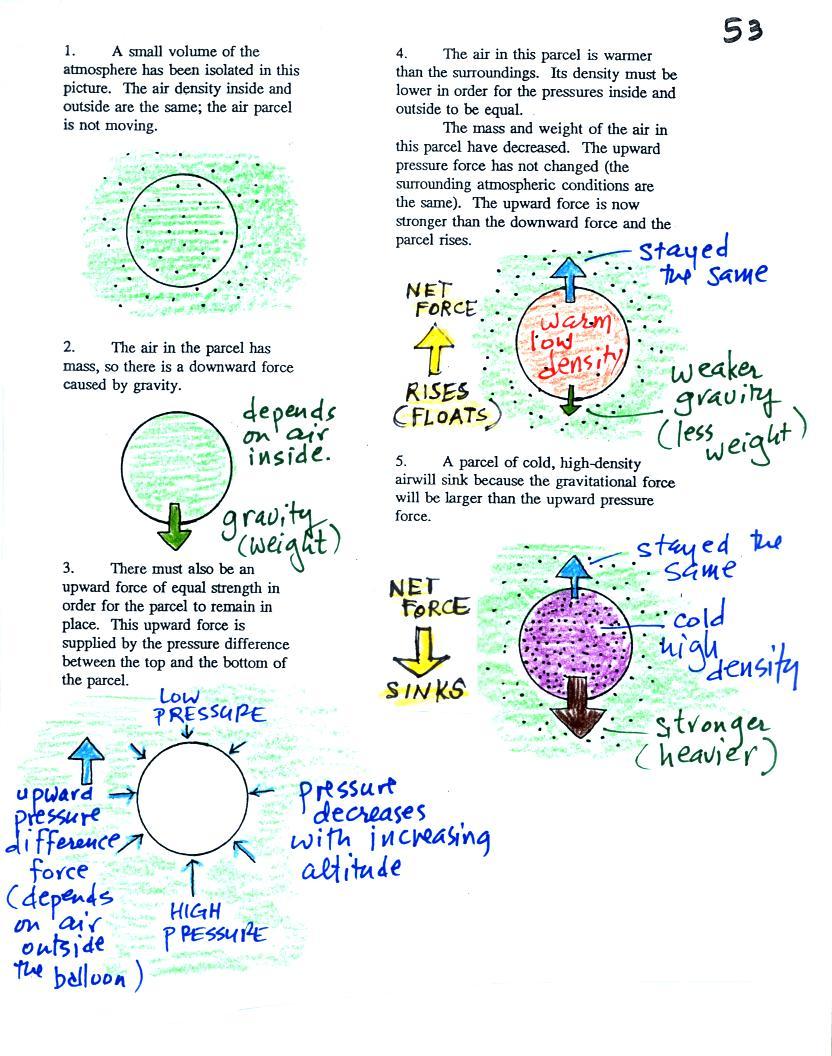
Step #3 Vertical forces acting
on parcels of air
And finally the last step toward
understanding why warm air rises and cold air sinks. We'll
have a look at the forces that act on parcels of air in the
atmosphere. This is something we have already
covered. The information below is found on p. 53 in the
photocopied ClassNotes.
Basically it comes down to this - there are two forces acting on a
parcel of air in the
atmosphere. They are shown on the left hand side
of the figure above.
The first force is gravity, it pulls downward. The strength
of the gravity force (the weight of the air in the parcel) depends
on the mass of the air inside
the parcel.
Second there is an upward pointing pressure difference
force. This force is caused by the air outside (surrounding)
the parcel. Pressure decreases with increasing
altitude. The pressure of the air at the bottom of a parcel
pushing upward is slightly stronger than the pressure of the air
at the top of the balloon that is pushing downward. The
overall effect is an upward pointing force.
When the air inside a parcel is exactly the same as the air
outside, the two forces are equal in strength and cancel
out. The parcel is neutrally buoyant and it wouldn't rise or
sink, it would just sit in place.
Now have a look at the right hand side of the figure.
If you replace the air inside the balloon with warm low density
air, it won't weigh as much. The gravity force is
weaker. The upward pressure difference force doesn't change
(because it is determined by the air outside the balloon which
hasn't changed) and ends up stronger than the gravity force.
The balloon will rise.
Conversely if the air inside is cold high density air, it
weighs more. Gravity is stronger than the upward pressure
difference force and the balloon sinks.
It all comes down to how the density of the in parcel compares
to the density of the air surrounding the parcel. If the
parcel is filled with low density air it will rise. A parcel
full of high density air will sink.
We did a short demonstration to show how density can
determine whether an object or a parcel of air will rise or
sink.
Convection demonstration
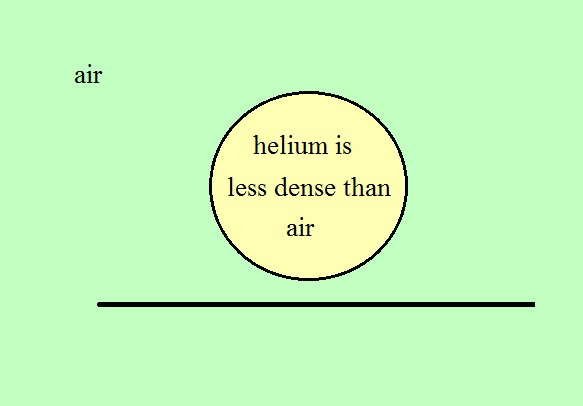
We used balloons
filled with helium (see bottom of p. 54 in the photocopied
Class Notes). Helium is less dense than air even when
it has the same temperature as the surrounding air.
The downward gravity force (weight of the helium filled
balloon) is weaker than the upward pressure difference
force. A helium-filled balloon doesn't need to warmed
up in order to rise.
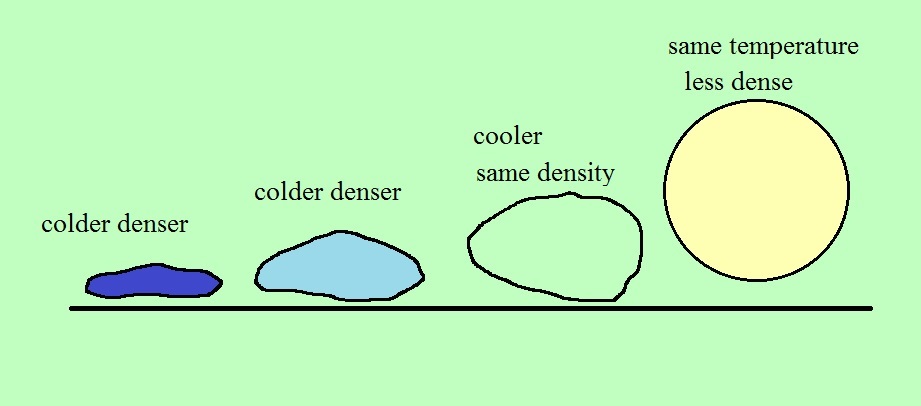
We dunk the helium filled balloon in liquid
nitrogen to cool it off and make it denser than air.
When you pull the balloon out of the liquid nitrogen the
helium is cold and denser than the surrounding air. I
set it on the table (dark blue above).
As the balloon of helium warms and expands its density
decreases (light blue). For a brief moment it has the
same density as the surrounding air (green). It's
neutrally buoyant at this point. Then it warms back to
near room temperature where it is again less dense than the
air and lifts off the table (yellow).
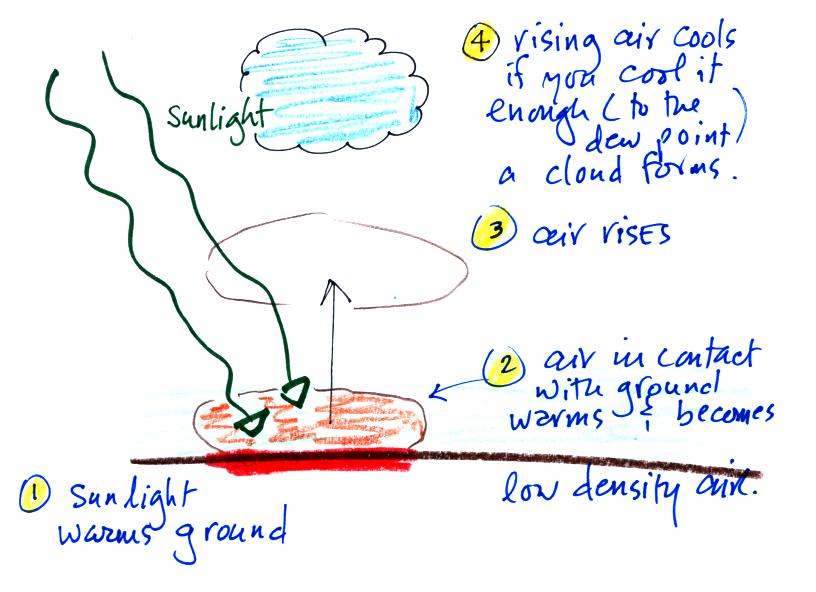
Free convection
Something like this happens in the atmosphere. I didn't
show the following picture in class. I
have another demonstration that I'll bring on Thursday and will
mention free convection then.
Sunlight shines through the atmosphere. Once it reaches
the ground at (1) it is absorbed and warms the ground.
This in turns warms air in contact with the ground (2) As
this air warms, its density starts to decrease. When the
density of the warm air is low enough, small "blobs" of air
separate from the air layer at the ground and begin to rise,
these are called "thermals." (3) Rising air expands and
cools (we've haven't covered this yet and it might sound a
little contradictory). If it cools enough (to the dew
point) a cloud will become visible as shown at Point 4.
This whole process is called convection; many of our summer
thunderstorms start this way.
Archimedes' principle
Here's another way of trying to understand why warm air
rises and cold air sinks - Archimedes Law or Principle. It's
a perhaps simpler way of understanding the topics. A
bottle of water can help you to visualize the law.
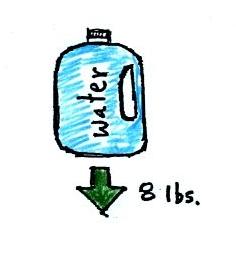
A gallon of water weighs about 8
pounds (lbs). I wouldn't want to carry that much water on
a hike unless I really thought I would need it.
If you submerge the gallon jug of water in a swimming pool,
the jug becomes, for all intents and purposes, weightless.
That seems kind of amazing. Archimedes' Law (see figure
below, from p. 53a in the photocopied ClassNotes) explains why
this is true.
Archimedes first of all tells you that the surrounding fluid
will exert an upward pointing buoyant force on the submerged
water bottle. That's why the submerged jug can become
weightless.
Archimedes law also tells you how to figure out how strong the
buoyant force will be. In this case the 1
gallon bottle will displace 1 gallon of pool water. One
gallon of pool water weighs 8 pounds. The upward buoyant
force will be 8 pounds, the same as the downward force.
The two forces are equal and opposite.
What Archimedes law doesn't really tell you is what causes
the upward buoyant force. You should know what the force
is - it's the upward pressure difference force.
|
|
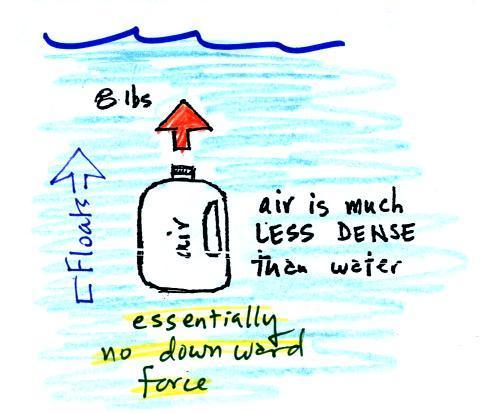
| We've poured out the water and filled
the 1 gallon jug with air. Air is much less dense
than water; compared to water, the jug will weigh
practically nothing. But it still displaces a gallon
of water and experiences the 8 lb. upward buoyant
force. The bottle of air would rise (actually it
shoots up to the top of the pool). |
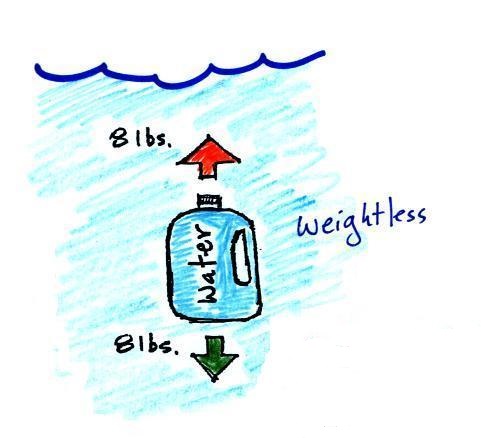
A bottle filled with water is
weightless. The density of the material inside and
outside the bottle are the same. |
I wish I could get my hands on a gallon
of mercury but I can't (and am not sure I'd be able to carry
it to class even if I could)
|
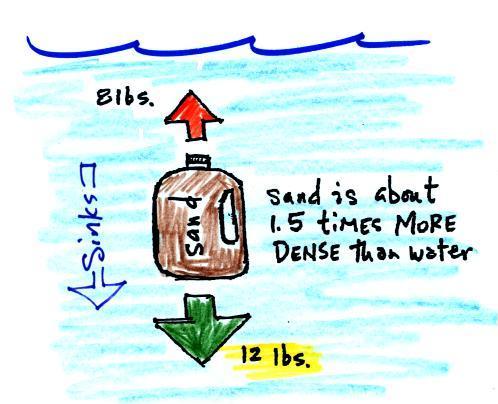
 |
| Sand is about 50% denser than
water. The weight of a gallon of sand is more
than a gallon of water. The downward force is
greater than the upward force and the bottle of sand
sinks. |
A gallon of water immersed in water
is weightless.
|
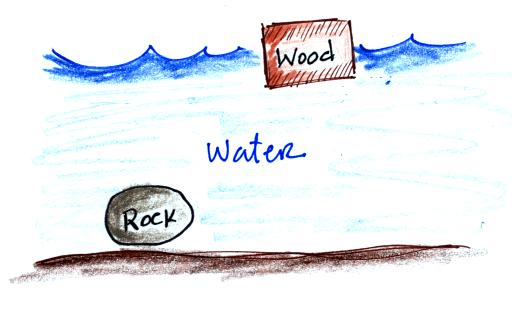
You can sum all of this up by saying anything that is
less dense than water will float in water,
anything that is more dense than water will sink in water.
Most types of wood will float (ebony and ironwood
will sink). Most rocks sink (pumice is an exception).
The same reasoning applies to air in the atmosphere though
it's harder to appreciate because air is invisible.
When we say immersed in a fluid the fluid can be a liquid
like water or a gas like air.
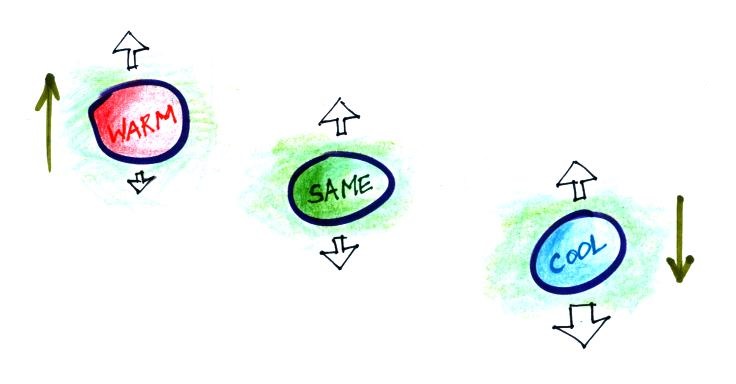
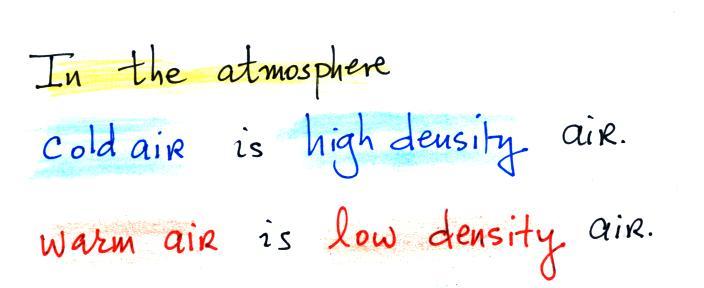
Air that is less dense (warmer) than the air around it will
rise. Air that is more dense (colder) than the air around
it will sink.
Here's a little more
information about Archimedes that I didn't mention in class.
There's a colorful demonstration that shows how small
differences in density can determine whether an object floats or
sinks.

A can of regular Pepsi was
placed in a beaker of water. The can should sink (it
didn't in class). A can of Diet Pepsi on the other hand
floated.
Both cans are made of aluminum which has a density almost
three times higher than water; aluminum by itself would
sink. The drink itself is largely water. The
regular soda also has a lot of high-fructose
corn syrup, the diet soda doesn't. The mixture of
water and corn syrup has a density greater than plain
water. There is also a little air (or perhaps carbon
dioxide gas) in each can (the diet soda probably wouldn't
float if it weren't for the gas in the can.
The average density of the can of regular soda (water &
corn syrup + aluminum + air) should end up being
slightly greater than the density of water. The average
density of the can of diet soda (water + aluminum + air) is
slightly less than the density of water.
In some respects people in swimming pools are like cans of
regular and diet soda. Some people float (they're a
little less dense than water), other people sink (slightly
more dense than water).

Here's another demonstration of what we
have been learning - a Galileo thermometer. It's a
little fragile and I already had too much stuff with me today
so I'll bring it (and explain how it works) on Thursday.

The great age of
stratospheric exploration
Some history during the last 15 minutes or so of
class. Pages 31 and 32 in the ClassNotes list some
of the significant events in the early study and
exploration of the atmosphere. A few of them are
included below.
Note the mercury barometer was invented in 1643.
The earliest balloon trips into the upper
atmosphere were in unheated and unpressurized gondolas.
Climbers have made it to the summit of Mt. Everest without
carrying supplementary oxygen but it is difficult and requires
acclimation. You can't acclimate to conditions above
25,000 ft and can't remain up there very long - it's referred
to as the "death zone." (Read "Into Thin
Air" by Jon Krakauer if you'd like to get some idea of
what it's like trying to climb Mt. Everest)
Note the clothing that Capt. Grey had to
wear to try to stay warm. All of his trips were in an
open, unpressurized gondola.

Source
of the image below

|

I believe this is the gondola flown into the
stratosphere by Auguste Piccard and Paul Kipfer is shown
above (source).
The figure caption is in German so I am not sure that is
the case.
|
Auguste
Piccard is shown in the figure at left. The
gondola he took into the stratosphere is shown at
right. Note how one side is black and the other
white. By turning the gondola they could control the
temperature inside (pointing the black side toward the sun
would warm the gondola, turning the white side would allow
the gondola to cool off).
We watched about 10 minutes of video describing
Piccard's first trip into the stratosphere (they very
nearly didn't make it back down alive).
That was about all the time we
had in class today. I
have several more videos that I would like to show at some
point.
You
might have heard about Felix
Baumgartner and the Red Bull Stratos balloon (or
seen the GoPro commercial during a recent Super
Bowl). On Oct. 14, 2012 he reached an altitude of
nearly 128,000 feet (39 km or 24 miles) and then
jumped. He reached a speed of 843 MPH on the way
down (Mach 1.25 or 1.25 times the speed of sound).
Here's a short
video (1:25) that I'll show in class on Thursday.
It shows portions of his jump. If you have time you
should really watch the longer
version (9:32). Baumgartner began to
spin during the descent but was able get out of it. He
came very close to blacking out.
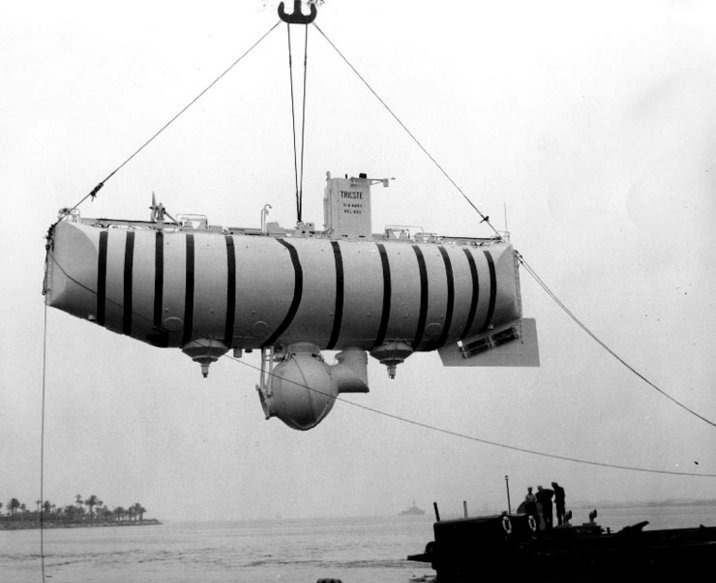
Jacques Piccard,
Auguste's son, would later travel
with Lt. Don Walsh of the US Navy
to a depth of about 35,800 feet in
the ocean in the Mariana Trench
(Auguste participated in some of
the test descents to 10,000
ft). They did that in the
Bathyscaph Trieste (shown below)
on Jan. 23, 1960 (source
of the image).
I'll try to show a short video of one of their test dives
(to 10,000 ft.)
Here's a National
Geographic video describing film director James
Cameron's much more recent dive to the Challenger Deep
in the Mariana Trench on Mar. 12, 2012 (2:16).
(note mention of the 16,000 psi
pressure on the submersible at the bottom of the ocean)
Bertrand
Piccard, Jacques' son (Auguste's grandson) was
part of the first two man team to circle the globe
non-stop in the Breitling Orbiter 3 balloon (Mar. 20,
1999). Brian Jones was the second team member
(source
of the left image above,
source
of the right image
). I've
got a pretty good video summary of their trip. Here
are three online videos of the event: short
summary (1:40),
longer
summary (6:15 with music only, no commentary)
and a full
documentary (54:06).