
Sea level pressure, 14.7 psi, might not
sound like much. But when you start to multiply 14.7
by all the square inches on your body it turns into a lot of
pounds of force. The yellow box on the person's chest
in the picture is a brick size, 4" x 8" = 32 square inch,
area. If you multiply 14.7 psi by 32 sq. in. you get
470 pounds! It would take a stack of 90 to 100 bricks
to produce that much weight.
Why isn't the person in the picture above crushed by the weight of the atmosphere above. The answer is that the person's body pushes back with the same amount of force. Air does the same thing. This is the topic we will explore next.
The upward (and sideways) force of air pressureWhy isn't the person in the picture above crushed by the weight of the atmosphere above. The answer is that the person's body pushes back with the same amount of force. Air does the same thing. This is the topic we will explore next.
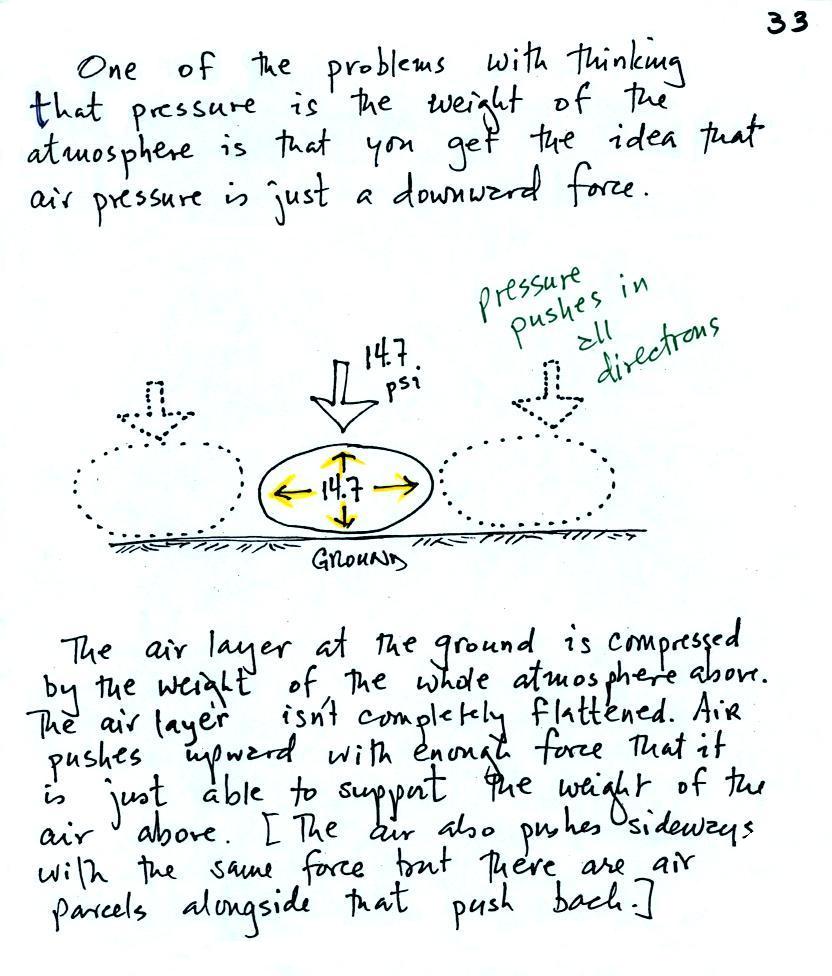
Air pressure is a force
that pushes downward, upward, and sideways. If you fill
a balloon with air and then push downward on it, you can feel
the air in the balloon pushing back (pushing upward).
You'd see the air in the balloon pushing sideways as
well.
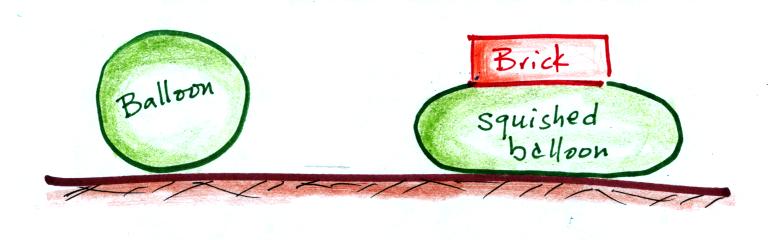
We were able to see this by placing a brick on top of a balloon. The balloon gets squished (pushed out sideways) but not flattened. It eventually pushes upward with enough force to support the brick. The squished balloon is what air at the bottom of the atmosphere looks like. And it is supporting more than just one brick, it is supporting a pile 90 to 100 bricks tall (just like the yellow box on the chest of the guy at the beach).
Another helpful representation of air in the atmosphere might be a people pyramid.
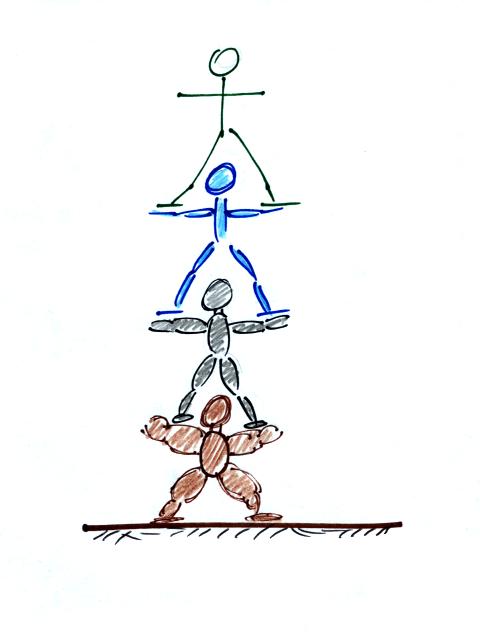
The people in the figure are like layers
of air in the atmosphere all stacked on top of each
other.
If the bottom person in the stack above were standing on a scale, the scale would measure the total weight of all the people in the pile. That's analogous to sea level pressure being determined by the weight of the all the air above.
The bottom person in the picture above must be strong enough to support the weight of all the people above. The bottom layer of the atmosphere pushes upward with enough pressure to support the weight of the air above.
Here's probably the most amazing example of air pressure pushing upward.
If the bottom person in the stack above were standing on a scale, the scale would measure the total weight of all the people in the pile. That's analogous to sea level pressure being determined by the weight of the all the air above.
The bottom person in the picture above must be strong enough to support the weight of all the people above. The bottom layer of the atmosphere pushes upward with enough pressure to support the weight of the air above.
Here's probably the most amazing example of air pressure pushing upward.
My car sits on 4 tires, which are really
nothing more than balloons. The air pressure in the four
tires pushes upward with enough force to keep the
1000 or 2000 pound vehicle off the ground. The air
pressure also pushes downward, you'd feel it if the car ran
over your foot. The air also pushes sideways with a lot
of force; tires need to be strong to keep from exploding or
coming off the wheel.
This was a logical point to do a demonstration. A demo that tries to prove that air pressure really does push upward as well as downward. Not only that but that the upward force is fairly strong. The demonstration is summarized on p. 35a in the ClassNotes.
This was a logical point to do a demonstration. A demo that tries to prove that air pressure really does push upward as well as downward. Not only that but that the upward force is fairly strong. The demonstration is summarized on p. 35a in the ClassNotes.

It's pretty obvious that if you fill a balloon with a little water and let go it will drop. And most everyone in the class knows why (see below - I broken the figure on p. 35b into pieces for clarity).
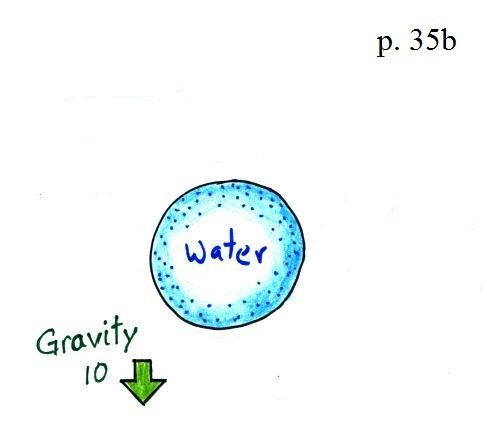
Gravity exerts a downward force on the balloon. I just made up a number, 10, to give you some idea of its strength. But the picture above isn't complete.
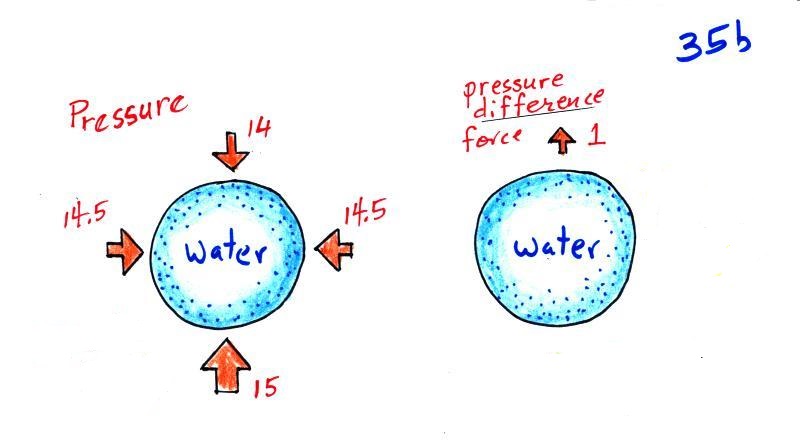
The water balloon is surrounded by air that is pushing upward, downward, and sideways on the balloon. These pressure forces are strong but mostly cancel each other out. The sideways forces do cancel out exactly.
The up and down forces aren't quite equal because pressure decreases with increasing altitude. The upward pointing force at the bottom is stronger (15 units) than the downward force at the top (14 units). They don't cancel and there is a weak upward pressure force (1 unit strong). I'm pretty sure that most people in the class don't know about this pressure difference force.
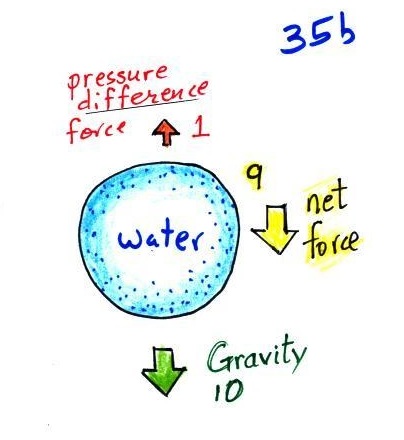
This picture includes all the forces. The downward gravity force is stronger than the upward pressure difference force and the balloon falls.
It seems like we could change things a little bit and somehow keep the upward and downward pressure forces from working against each other. We did that in a class demonstration.
An upward pressure force demonstration
In the demonstration a wine glass is filled with water (about the same amount of water that you might put in a small water balloon).
A small plastic lid is used to cover the wine glass (you'll need to look hard to see the lid in the photo above). The wine glass is then turned upside and the water does not fall out.

All the same forces are shown again in the
left most figure. We'll split that into two parts - a
water and lid part and an empty glass part.
The 14 units of pressure force is pushing on the glass now and not the water. I was holding onto the glass, I'm the one that balanced out this downward pressure force.
Gravity still pulls downward on the water with the same 10 units of force. But with 15 units, the upward pressure force is able to overcome the downward pull of gravity. It can do this because all 15 units are used to overcome gravity and not to cancel out the downward pointing pressure force.
The 14 units of pressure force is pushing on the glass now and not the water. I was holding onto the glass, I'm the one that balanced out this downward pressure force.
Gravity still pulls downward on the water with the same 10 units of force. But with 15 units, the upward pressure force is able to overcome the downward pull of gravity. It can do this because all 15 units are used to overcome gravity and not to cancel out the downward pointing pressure force.
The Magdeburg hemispheres
experiment (sideways pressure force)
Air pressure pushes downward with hundreds of pounds of force on someone lying on the beach.
The pressure of the air in tires pushes upward with enough force to keep a 1 ton automobile off the ground.
What about the sideways air pressure force?
Here's a description of the demonstration I'd like to try, it involves Magdeburg hemispheres and two teams of horses (the following quote and the figure below are from an article in Wikipedia):
" ... Magdeburg hemispheres are a pair of large copper hemispheres with mating rims, used to demonstrate the power of atmospheric pressure. When the rims were sealed with grease and the air was pumped out, the sphere contained a vacuum and could not be pulled apart by teams of horses. The Magdeburg hemispheres were designed by a German scientist and mayor of Magdeburg, Otto von Guericke in 1656 to demonstrate the air pump which he had invented, and the concept of atmospheric pressure."
Air pressure pushes downward with hundreds of pounds of force on someone lying on the beach.
The pressure of the air in tires pushes upward with enough force to keep a 1 ton automobile off the ground.
What about the sideways air pressure force?
Here's a description of the demonstration I'd like to try, it involves Magdeburg hemispheres and two teams of horses (the following quote and the figure below are from an article in Wikipedia):
" ... Magdeburg hemispheres are a pair of large copper hemispheres with mating rims, used to demonstrate the power of atmospheric pressure. When the rims were sealed with grease and the air was pumped out, the sphere contained a vacuum and could not be pulled apart by teams of horses. The Magdeburg hemispheres were designed by a German scientist and mayor of Magdeburg, Otto von Guericke in 1656 to demonstrate the air pump which he had invented, and the concept of atmospheric pressure."
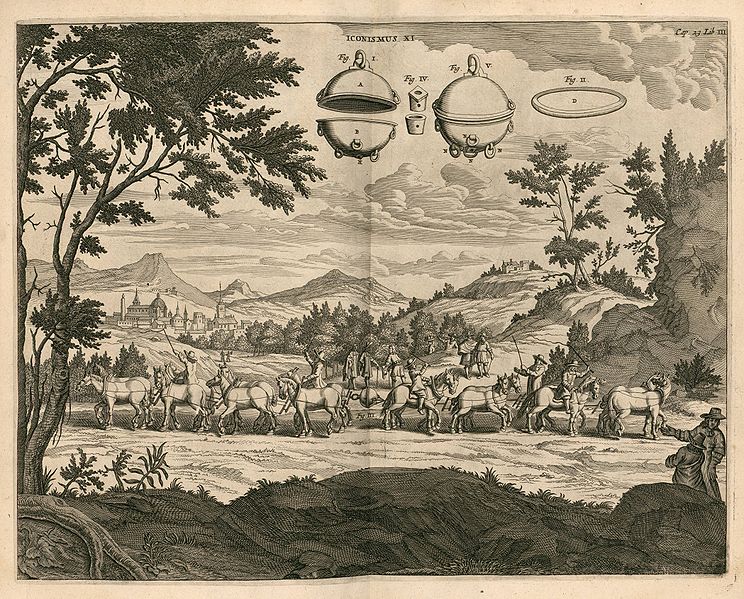
Gaspar Schott's sketch of Otto von Guericke's
Magdeburg hemispheres experiment (from the Wikipedia article
referenced above)
It is the pressure of the air pushing inward against the outside surfaces of the hemispheres that keeps them together. The hemispheres appear to have had pretty large surface area. There would be 15 pounds of force pressing against every square inch (at sea level) of the hemisphere which could easily have been several thousand pounds of total force.
I would like to see the demonstration staged in Arizona Stadium at halftime during a home football game.
Suction cups work the same way

The suction cup has been pressed against smooth surface. The cup is flexible and can be pulled away from the wall leaving a small volume between the wall and the cup where there isn't any air (a vacuum). There's no air pressure pushing outward on the suction cup, just pressure from the air surrounding the suction cup that is pushing inward.
Changes in air density with altitude
We've spent a lot of time (too much?) looking at air pressure and how it changes with altitude. Next we'll consider air density and air temperature.
How does air density change with increasing altitude? You should know the answer to that question. You get out of breath more easily at high altitude than at sea level. Air gets thinner (less dense) at higher altitude. A lungful of air at high altitude just doesn't contain as many oxygen molecules as it does at lower altitude or at sea level.
It would be nice to also understand why air density decreases with increasing altitude.
 |
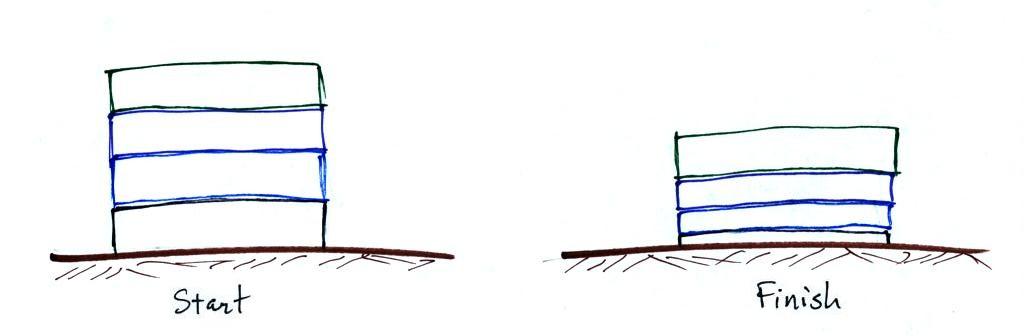 |
The people pyramid reminds you that there is more weight, more pressure, at the bottom of the atmosphere than there is higher up.
Layers of air are not solid and rigid like in a stack of bricks. Layers of air are more like mattresses stacked on top of each other. Mattresses are compressible, bricks (and people) aren't. Mattresses are also reasonably heavy, the mattress at the bottom of the pile would be squished by the weight of the three mattresses above. This is shown at right. The mattresses higher up aren't compressed as much because there is less weight remaining above. The same is true with layers of air in the atmosphere.

The statement above is at the top of p. 34 in the photocopied ClassNotes. I've redrawn the figure found at the bottom of p. 34 below.
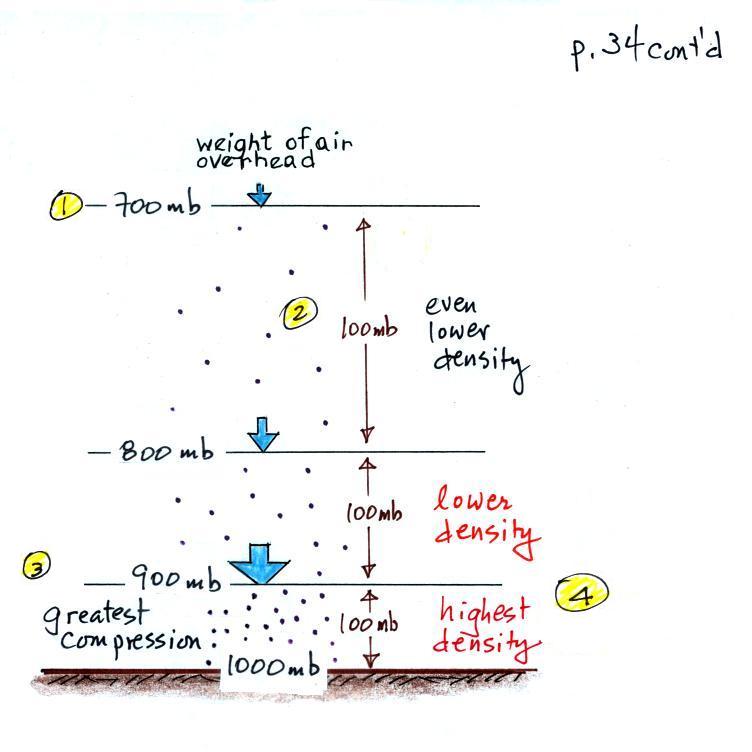
There's a surprising amount of information in this figure and it is worth spending a minute or two looking for it and thinking about it.
1. You can first notice and remember that pressure decreases with increasing altitude. 1000 mb at the bottom decreases to 700 mb at the top of the picture. You should be able to explain why this happens.
2. Each layer of air contains the same amount (mass) of air. This is a fairly subtle point. You can tell because the pressure drops by the same amount, 100 mb, as you move upward through each layer. Pressure depends on weight. So if all the pressure changes are equal, the weights of each of the layers must be the same. Each of the layers must contain the same amount (mass) of air (each layer contains 10% of the air in the atmosphere).
3. The densest air is found at the bottom of the picture. The bottom layer is compressed the most because it is supporting the weight of all of the rest of the atmosphere. It is the thinnest layer in the picture and the layer with the smallest volume. Since each layer has the same amount of air (same mass) and the bottom layer has the smallest volume it must have the highest density. The top layer has the same amount of air but about twice the volume. It therefore has a lower density (half the density of the air at sea level). Density is decreasing with increasing altitude. That's the main point in this figure.
4. Finally pressure is decreasing most rapidly with increasing altitude in the densest air in the bottom layer. We'll make use of this concept again at the end of the semester when we try to figure out why/how hurricanes intensify and get as strong as they do.
Principle of the mercury barometer

One of the more impressive seesaws (teeter totters) that I've seen (source of this image). If you understand how this works you'll be able to figure out how barometers function.
A mercury barometer is used to measure atmospheric pressure and is really just a balance that can be used to weigh the atmosphere.
 |
 |
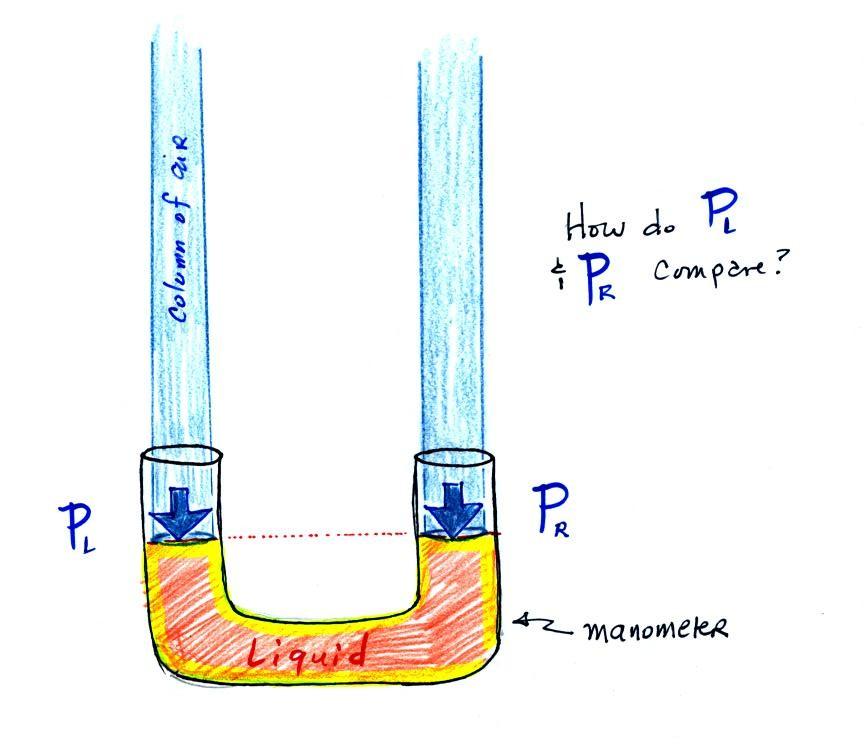
The instrument in the left figure above ( a u-shaped glass tube filled with a liquid of some kind) is actually called a manometer and can be used to measure pressure difference. The two ends of the tube are open so that air can get inside and air pressure can press on the liquid. Given that the liquid levels on the two sides of the manometer are equal, what could you say about PL and PR?
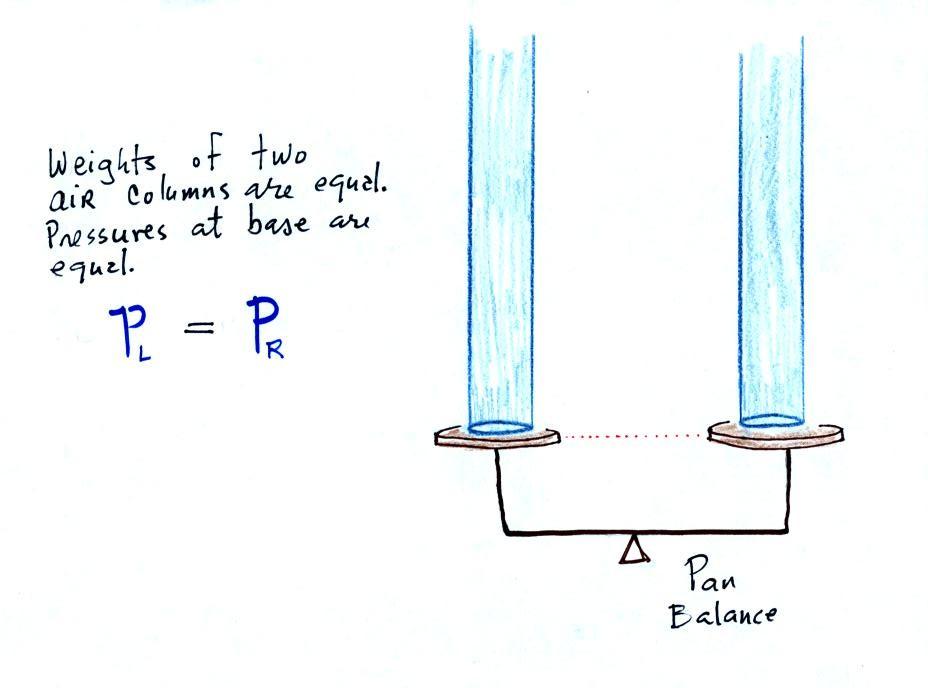
The liquid can slosh back and forth just like the pans on a balance can move up and down. A manometer really behaves just like a pan balance (pictured above at right) or a teeter totter (seesaw). Because the two pans are in balance, the two columns of air have the same weight. PL and PR are equal. Note: you don't really know what either pressure is, just that they are equal).
 |
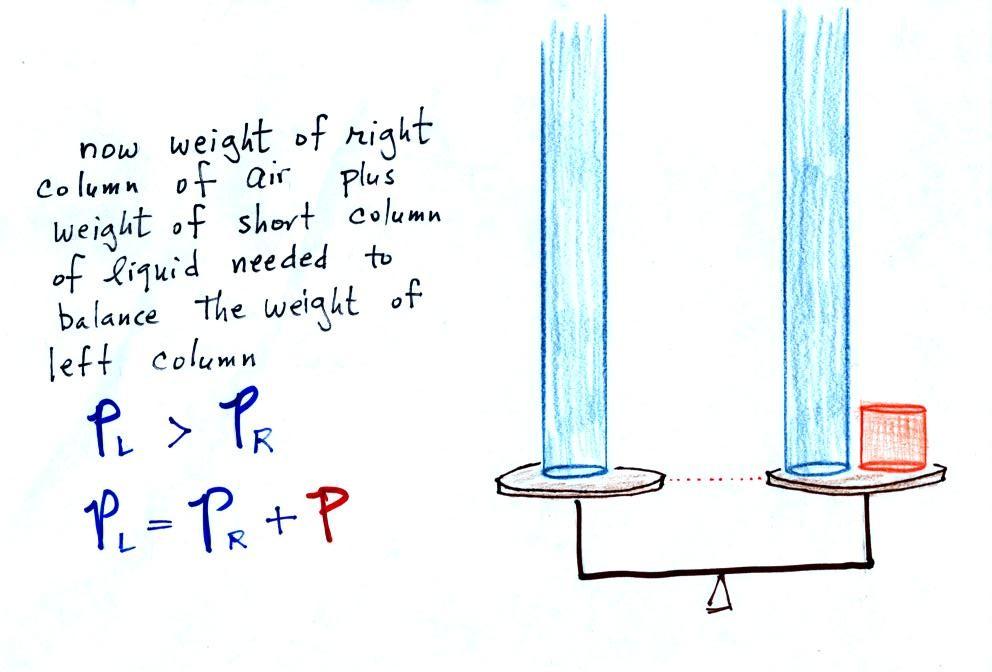 |
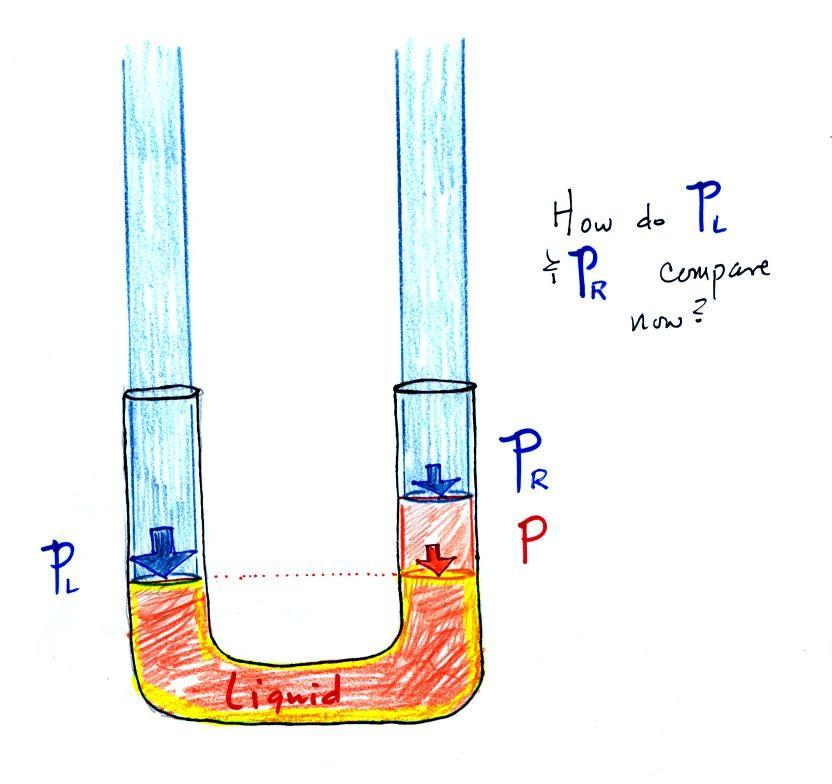
Now the situation is a
little different, the liquid levels are no longer
equal. You probably realize that the air pressure on
the left, PL, is a
little higher than the air pressure on the right, PR. PL is now being balanced
by PR + P acting together. P is the pressure produced by
the weight of the extra fluid on the right hand side of the
manometer (the fluid that lies above the dotted line).
The height of the column of extra liquid provides a
measure of the difference between PL and PR.
Next we will just go and close off the right hand side of the manometer.
Next we will just go and close off the right hand side of the manometer.
 |
 |
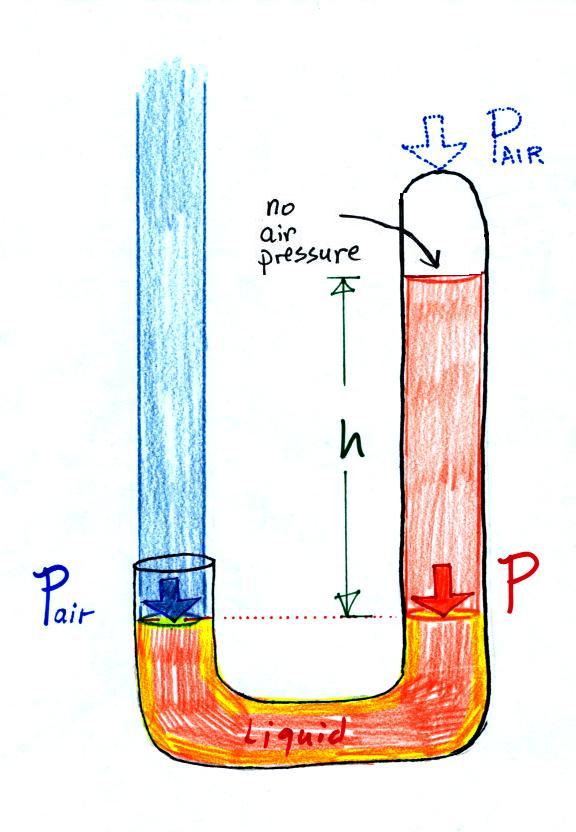
Air pressure can't get
into the right tube any more. Now at the level of
the dotted line the balance is between Pair and P (pressure by the extra
liquid on the right). If Pair changes, the height
of the right column, h, will change. You now
have a barometer, an instrument that can measure and
monitor the atmospheric pressure.
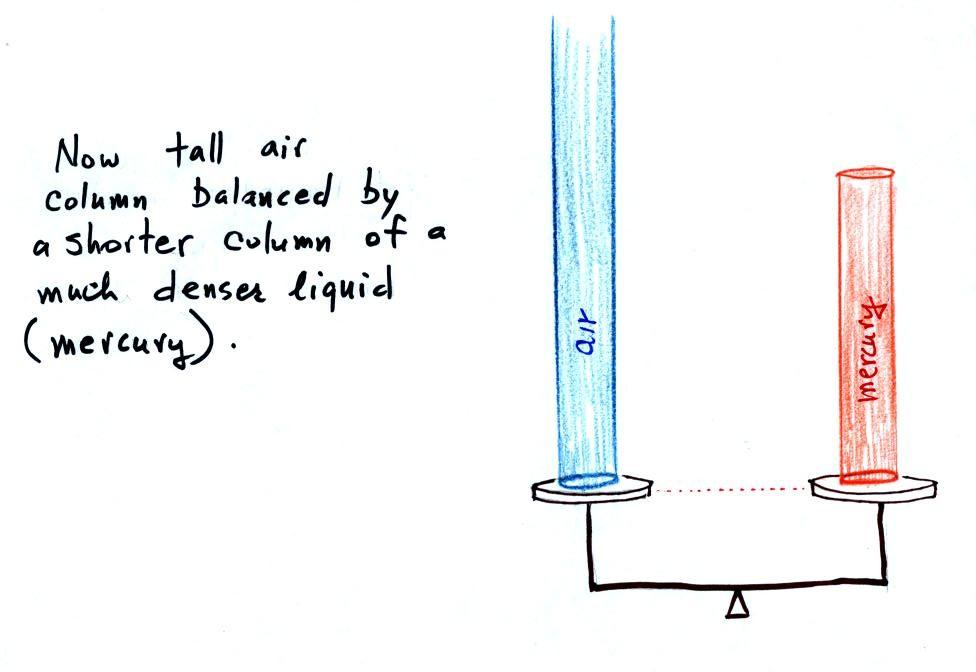
Barometers like this are usually filled with mercury. Mercury is a liquid. You need a liquid that can slosh back and forth in response to changes in air pressure. Mercury is also very dense which means the barometer won't need to be as tall as if you used something like water. A water barometer would need to be over 30 feet tall. With mercury you will need only a 30 inch tall column to balance the weight of the atmosphere at sea level under normal conditions (remember the 30 inches of mercury pressure units mentioned earlier). Mercury also has a low rate of evaporation so you don't have much mercury gas at the top of the right tube (there's some gas, it doesn't produce much pressure, but it would be hazardous you if you were to start to breath it).
Barometers like this are usually filled with mercury. Mercury is a liquid. You need a liquid that can slosh back and forth in response to changes in air pressure. Mercury is also very dense which means the barometer won't need to be as tall as if you used something like water. A water barometer would need to be over 30 feet tall. With mercury you will need only a 30 inch tall column to balance the weight of the atmosphere at sea level under normal conditions (remember the 30 inches of mercury pressure units mentioned earlier). Mercury also has a low rate of evaporation so you don't have much mercury gas at the top of the right tube (there's some gas, it doesn't produce much pressure, but it would be hazardous you if you were to start to breath it).
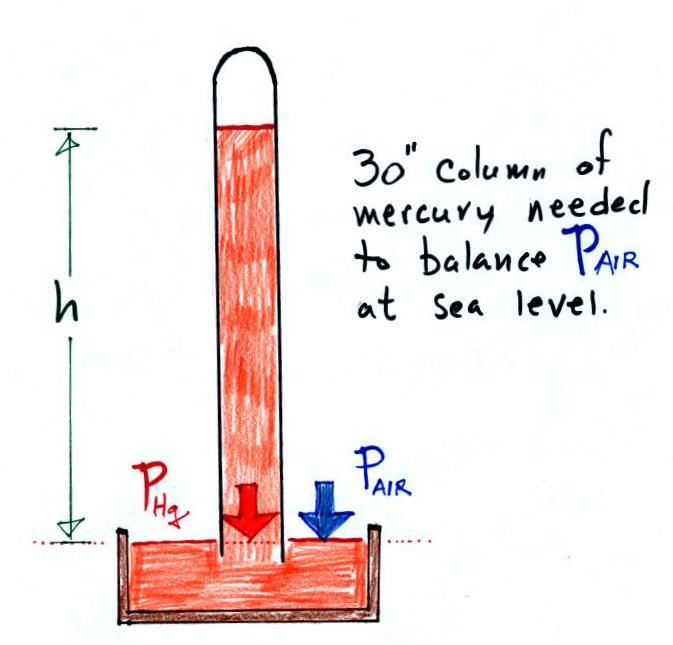
Here is a more conventional barometer design. The bowl of mercury is usually covered in such a way that it can sense changes in pressure but is sealed to keep poisonous mercury vapor from filling a room.
Average and extreme sea level pressure values
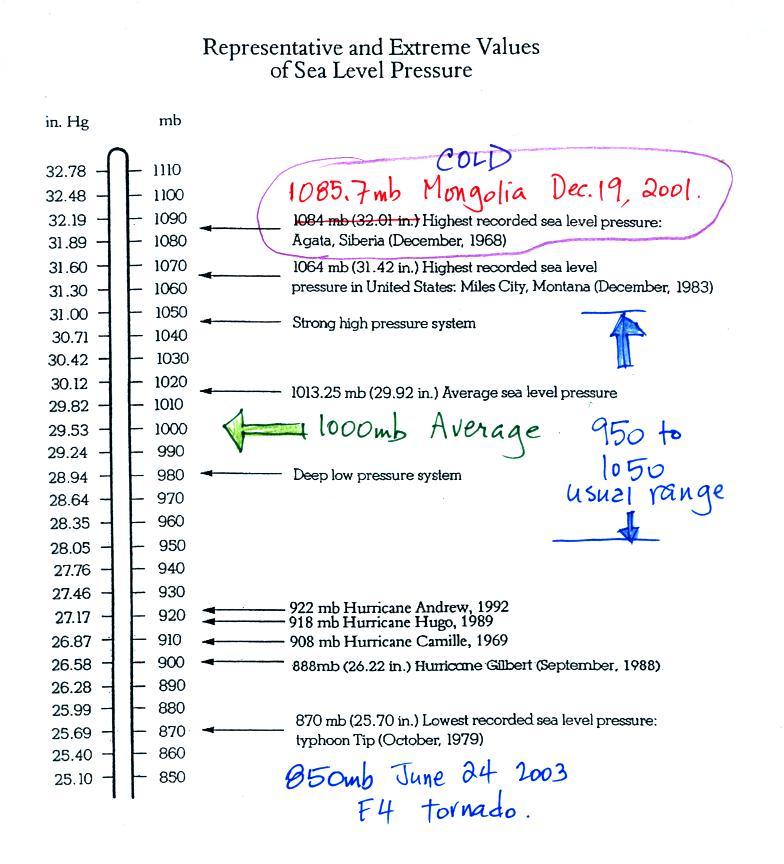
Average sea level atmospheric pressure is about 1000 mb. The figure above (p. 30 in the photocopied Class Notes) gives 1013.25 mb but 1000 mb is close enough in this class. The actual pressure can be higher or lower than this average value and usually falls between 950 mb and 1050 mb.
The figure also includes record high and low pressure values. Record high sea level pressure values occur during cold weather. The TV weather forecast will often associate hot weather with high pressure. They are generally referring to upper level high pressure (high pressure at some level above the ground) rather than surface pressure. There is some question about the accuracy of the 1085.7 mb value above. The problem is that the pressure was measured at over 5000 feet altitude and a calculation was needed to figure out what the pressure would have been if the location were at sea level. That calculation can introduce uncertainty. But you don't really need to be concerned with all that, I just wanted to give you an idea of how high sea level pressure can get.
Most of the record low pressure values have all been set by intense hurricanes. Hurricane Wilma in 2005 set a new record low sea level pressure reading for the Atlantic, 882 mb. Hurricane Katrina had a pressure of 902 mb. The following table lists some of the information on hurricane strength from p. 146a in the photocopied ClassNotes. 2005 was a very unusual year, 3 of the 10 strongest N. Atlantic hurricanes ever occurred in 2005.
| Most
Intense North Atlantic Hurricanes |
Most
Intense Hurricanes to hit the US Mainland |
| Wilma
(2005) 882 mb Gilbert (1988) 888 mb 1935 Labor Day 892 mb Rita (2005) 895 mb Allen (1980) 899 Katrina (2005) 902 |
1935
Labor Day 892 mb Camille (1969) 909 mb Katrina (2005) 920 mb Andrew (1992) 922 mb 1886 Indianola (Tx) 925 mb |
What makes hurricanes so intense is the pressure gradient, i.e. how quickly pressure changes with distance (horizontal distance). Pressure can drop from near average values (1000 mb) at the edges of the storm to the low values shown above at the center of the storm. This large pressure gradient is what causes the strong winds found in a hurricane.
The 850 mb pressure value measured in 2003 inside a strong tornado in Manchester, South Dakota (F4 refers to the Fujita scale rating, F5 is the highest level on the scale). This is very difficult (and very dangerous) thing to try to do. Not only must the instruments be built to survive a tornado but they must also be placed on the ground ahead of an approaching tornado and the tornado must then pass over the instruments (also the person placing the instrument needs to get out of the way of the approaching tornado).
You can experience much lower pressure values than shown above (roughly 700 mb) by just driving up to Mt. Lemmon.
Air temperature changes with altitude, troposphere & stratosphere
What happens to air temperature with increasing altitude. Again our personal experience is that it decreases with increasing altitude. It is colder at the top of Mt. Lemmon than it is here in the Tucson valley.
That is true up to an altitude of about 10 km (about 30,000 ft.). People were very surprised in the early 1900s when they used balloons to carry instruments above 10 km and found that temperature stopped decreased and even began to increase with increasing altitude.

Measurements of air temperature at high altitude in unmanned balloons lead to the discovery of the stratosphere in about 1900 (the information above is on p. 31 in the ClassNotes).
The figures below are more clearly drawn versions of what was done in class.
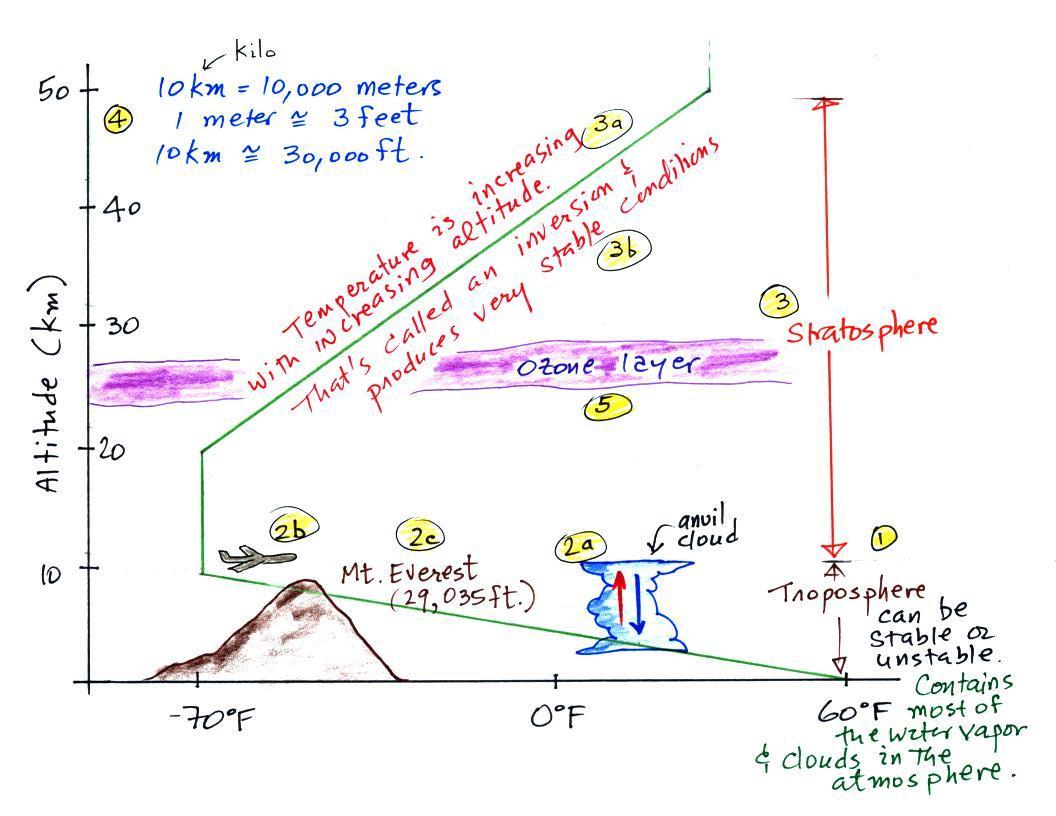
The atmosphere can be split into layers depending on whether temperature is increasing or decreasing with increasing altitude. The two lowest layers are shown in the figure above. There are additional layers (the mesosphere and the thermosphere) above 50 km but we won't worry about them in this class.
1. We live in the troposphere. The troposphere is found, on average, between 0 and about 10 km altitude, and is where temperature usually decreases with increasing altitude. [the troposphere is usually a little higher in the tropics and lower at polar latitudes]
The troposphere can be stable or unstable (tropo means "to turn over" and refers to the fact that air can move up and down in the troposphere). The troposphere contains most of the water vapor in the atmosphere (the water vapor comes from evaporation of ocean water and then gets mixed throughout the troposphere by up and down air motions) and is where most of the clouds and weather occurs.
2a. The thunderstorm shown in the figure with its strong updrafts and downdrafts indicates unstable conditions. When the thunderstorm reaches the top of the troposphere, it runs into the bottom of the stratosphere which is a very stable layer. The air can't continue to rise into the stratosphere so the cloud flattens out and forms an anvil (anvil is the name given to the flat top of the thunderstorm). The flat anvil top is something that you can go outside and see and often marks the top of the troposphere.
|
|
|
Here are several images of thunderstorms and anvil clouds taken from above, from the International Space Station (all 3 images courtesy of the Image Science and Analysis Laboratory, NASA Johnson Space Flight Center, www.eol.jsc.nasa.gov).

This photo of Mt. Everest was selected
as the Picture of the Day on Wikipedia for Dec. 22, 2007.
Photo credit: Luca Galluzi www.galluzi.it
Photo credit: Luca Galluzi www.galluzi.it
2b. The summit of Mt. Everest is a little over 29,000 ft. tall and is close to the average height of the top of the troposphere.
2c. Cruising altitude in a passenger jet is usually between 30,000 and 40,000, near or just above the top of the troposphere, and at the bottom of the stratosphere. The next time you're in an airplane try to look up at the sky above. There's less air and less scattering of light. As a result the sky is a darker purple color not blue. If you get high enough the sky would eventually become black.
3. Temperature remains constant between 10 and 20 km and then increases with increasing altitude between 20 and 50 km. These two sections form the stratosphere. The stratosphere is a very stable air layer. Increasing temperature with increasing altitude is called an inversion. This is what makes the stratosphere so stable.
4. A kilometer is one thousand meters. Since 1 meter is about 3 feet, 10 km is about 30,000 feet. There are 5280 feet in a mile so this is about 6 miles (about is usually close enough in this class).
5. The ozone layer is found in the stratosphere. Peak ozone concentrations occur near 25 km altitude.
Here's the same picture drawn again (for clarity) with some additional information. We need to explain why when temperature decreases all the way up to the top of the troposphere, it can start increasing again in the stratosphere.
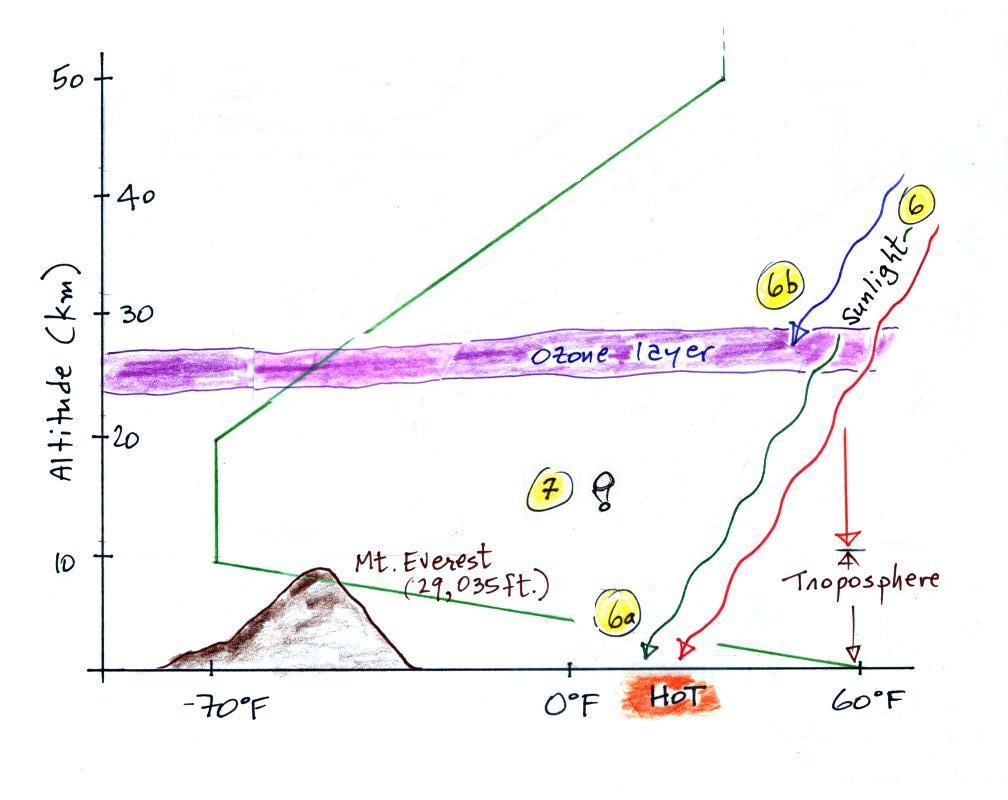
6. Sunlight is a mixture of ultraviolet (7%), visible (44%, colored green in the picture above) and infrared light (49%, colored red). We can see the visible light.
6a. On average about 50% of the sunlight arriving at the top of the atmosphere passes through the atmosphere and is absorbed at the ground (20% is absorbed by gases in the air, 30% is reflected back into space). This warms the ground. The air in contact with the ground is warmer than air just above. As you get further and further from the warm ground, the air is colder and colder. This explains why air temperature decreases with increasing altitude in the troposphere.
5b. How do you explain increasing temperature with increasing altitude in the stratosphere?
Absorption of ultraviolet light by ozone warms the air in the stratosphere and explains why the air can warm (oxygen also absorbs UV light). The air in the stratosphere is much less dense (thinner) than in the troposphere. So even though there is not very much UV light in sunlight, it doesn't take as much energy to warm this thin air as it would to warm denser air closer to the ground.
7. That's a manned balloon; Auguste Piccard and Paul Kipfer are inside. They were the first men to travel into the stratosphere (see pps 31 & 32 in the photocopied Class Notes). It really was quite a daring trip at the time, and they very nearly didn't survive it. I'll try to show a short video segment about their trip in the next week or two.