In our next lecture we will change directions and start covering
cloud
electrification.
First we need to do a little review and have a look at the structure of
a cold thunderstorm cloud. Cold refers
to the fact that much of the cloud is found at high enough altitude
that it is at below freezing
temperatures and contains ice crystals. This is the case for
thunderstorm clouds even in
Tucson on the hottest day of the summer.
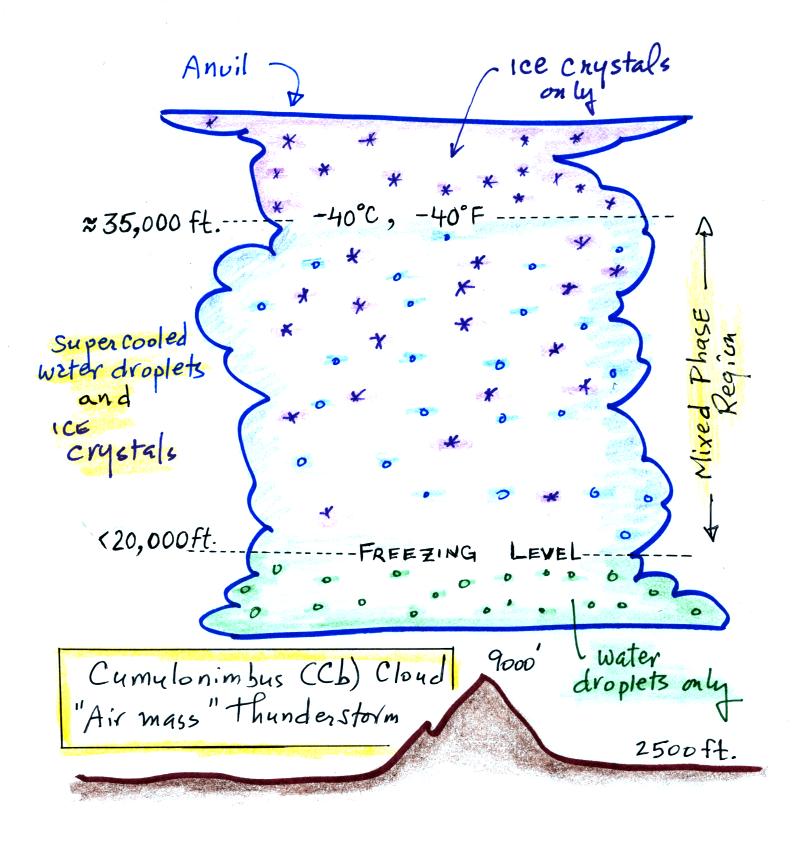
The cloud above is meant to be fairly typical of a summer thunderstorm
in S. Arizona. Cloud base is found above the top of Mt. Lemmon
(9000 ft. altitude). The freezing level is usually well below
20,000 feet altitude. The top of the cloud in this figure is
found between 35,000 and 40,000 feet (some stronger storms might get
higher than that).
The important part of the cloud, both for precipitation
formation and electrification, is the middle, mixed-phase region.
There you find both ice crystals and supercooled water droplets
(water droplets cooled to below freezing that are unable to freeze).
Before we
try to understand why it is difficult for water droplets to freeze it
might be worth noting that the formation of water droplets does
not occur as you might have
imagined. You might have thought that once the relative humidity
in the air (RH) reaches 100%
that water vapor would simply condense and form little droplets.
This is not the case; we will find that small particles in the air
called condensation play an essential role in cloud formation.
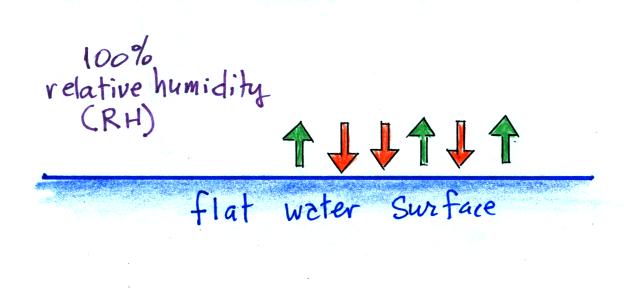
We'll first illustrate that when the air is saturated with water
vapor (the relative humidity is 100%) the rates of evaporation and
condensation above a flat surface of water will
be equal.
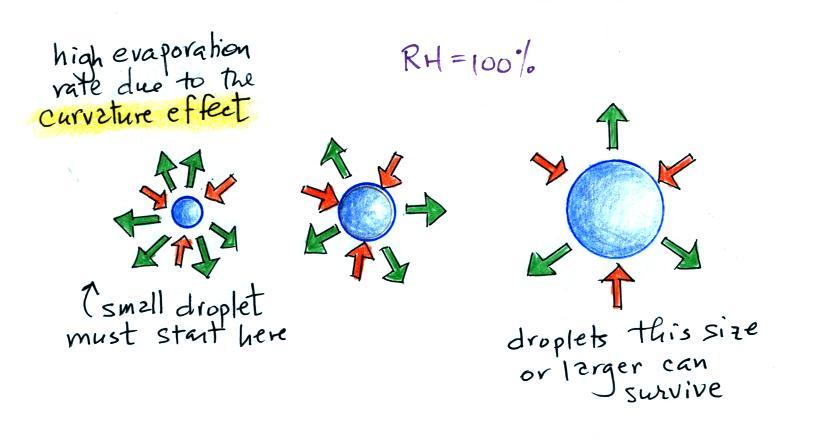
The rate of evaporation from a small droplet, however, is much
higher than
you would find over a flat surface of water This is called
the
curvature effect and is illustrated above (6 arrows of
evaporation from the smallest drop, 4 arrows from the
middle drop, and 3 arrows from the largest drop above which is the same
rate as you would find over a flat water surface). If
a small droplet of pure water were to form
it would quickly evaporate; condensation from the moist
surroundings would not be enough to overcome the high rate of
evaporation. A droplet must somehow reach a critical size before
it will be in
equilibrium with its surroundings.
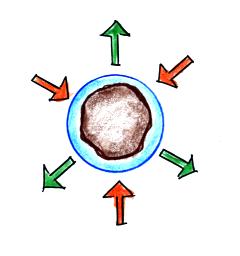
Particles in the air, cloud
condensation nuclei (CCN), make it much easier for cloud droplets
to form. Water vapor could simply condense onto a particle of
appropriate size as shown above. The water droplet could
effectively start at,
rather than grow to, the critical size and would be in equilibrium with
its surroundings.
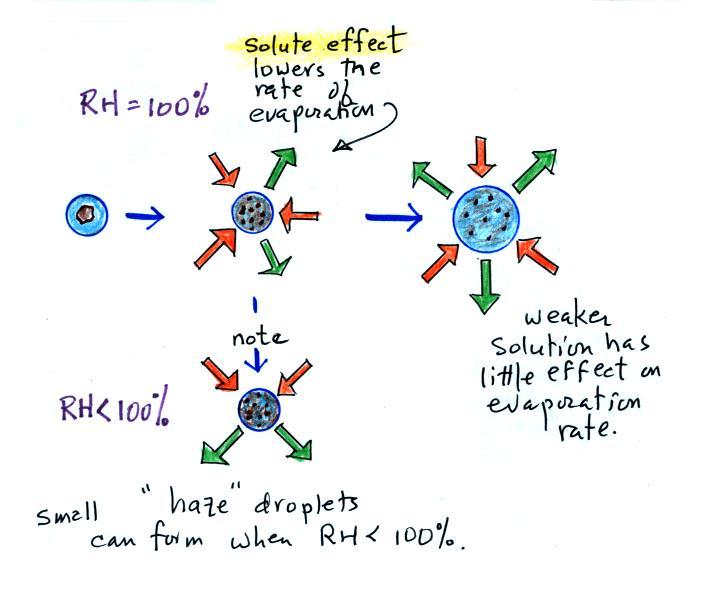
Note that it is possible for small droplets of
solution to form and be in equilibrium with their surroundings when the
relative humidity is less than 100%. These are called haze
droplets.
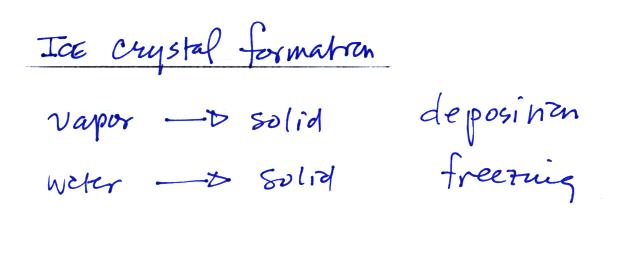
Ice crystals in a cloud can basically form in two ways
Water vapor can turn directly to ice or supercooled water droplets
can freeze. Just as was the case with condensation and the
formation of water droplets, the formation of ice crystals is much
easier if an "ice crystal" nucleus is involved.
The problem is that there aren't
many materials that can act as an ice nucleus.
Silver iodide is used in cloud seeding. Kaolinite is a clay
material (that was used at one time in Kaopectate for the treatment of
diarrhea, bismuch subsalicilate is now used). Certain bacteria
also are effective ice nuclei (bacteria are added to water in
snow-making operations at ski resorts to ensure that the water freezes
when sprayed onto the slopes).
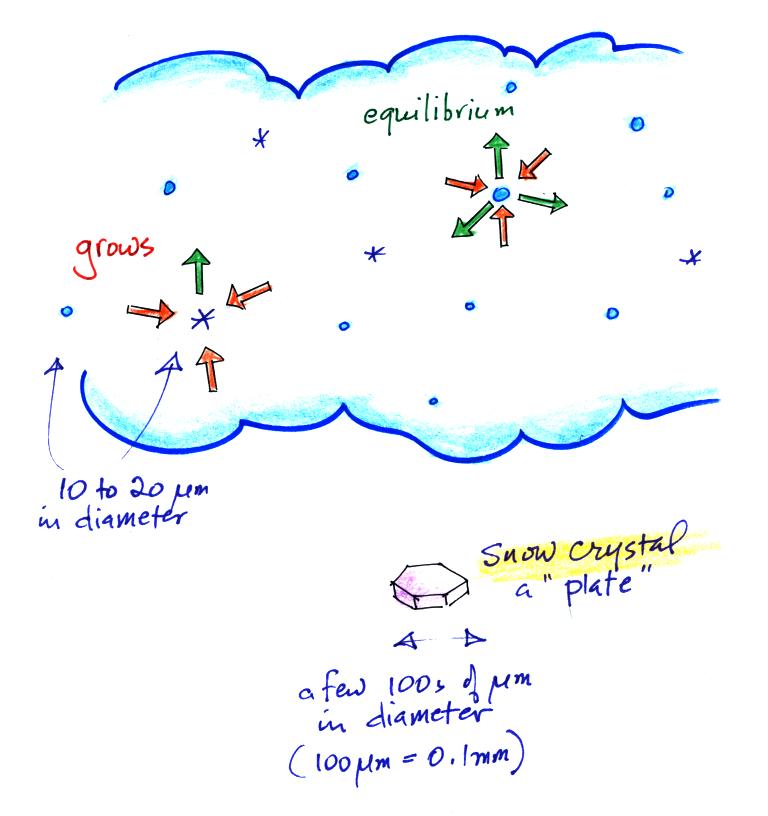
Once an ice crystals forms, it is able to grow relatively quickly
in the moist environment in the cloud even when the water droplets do
not. Ice crystals evaporate (actually they sublimate) at a slower
rate
than water droplets. Because the surrounding air is moist enough
to keep the water droplets in equilibrium (3 arrows of condensation
balancing 3 arrows of evaporation in the figure above), and because
water vapor will condense onto the ice crystals at the same rate, the
ice crystals will grow and become snow crystals (just bigger ice
crystals).
Snow crystals
come in lots of different shapes (called "habits", a plate is sketched
above) depending on the amount of moisture in the cloud and the
temperature. Have a look at
photomicrographs of some snow crystals at www.snowcrystals.com.
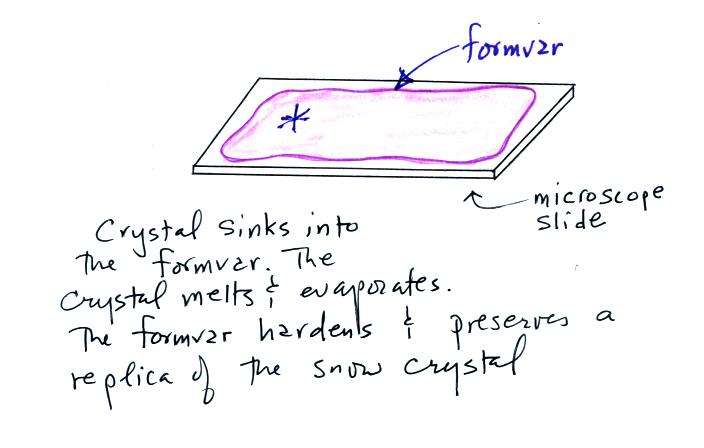
It used to be (and maybe still is) that people would make replicas
of snow crystals by allowing them to fall onto a microscope slide
coated with formvar (a plastic resin material of some kind dissolved in
acetone or something like that). The crystal would melt and
evaporate but would leave behind an impression in the formvar.
The acetone in the formvar would evaporate and the formvar would harden
leaving a permanent record of the snow crystal that could be examined
or photographed under a microscope.
A couple of more things you need to be familiar with before we start
talking about electrification processes.
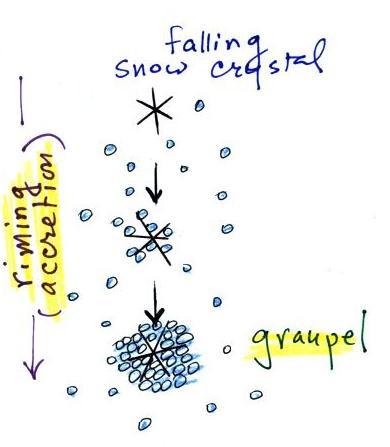
The snow crystal in the picture above is falling and colliding
with supercooled water droplets. The droplets stick and
freeze. This process is called riming or accretion. If this
goes on long enough the snow crystal can get completely covered with
frozen droplets. The resulting particle is called graupel, soft
hail, or snow pellets. Graupel particles can grow up to maybe 1/4
inch across. They have a frosty white appearance and resemble a
miniature snow ball.
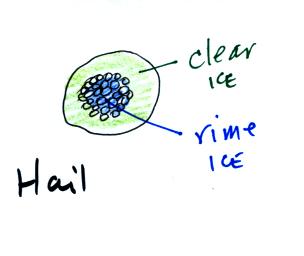
Graupel is really not hail. Hail usually starts with a
graupel core and then has alternating layers of clear ice and rime ice
(the frosty white ice that makes up graupel). In Tucson hail
usually has just a graupel core and a single layer of clear ice.
The appearance is quite distinctive and clearly different from
graupel. In the big severe thunderstorms in the Central Plains
the hailstones can have many layers of rime ice and clear ice.
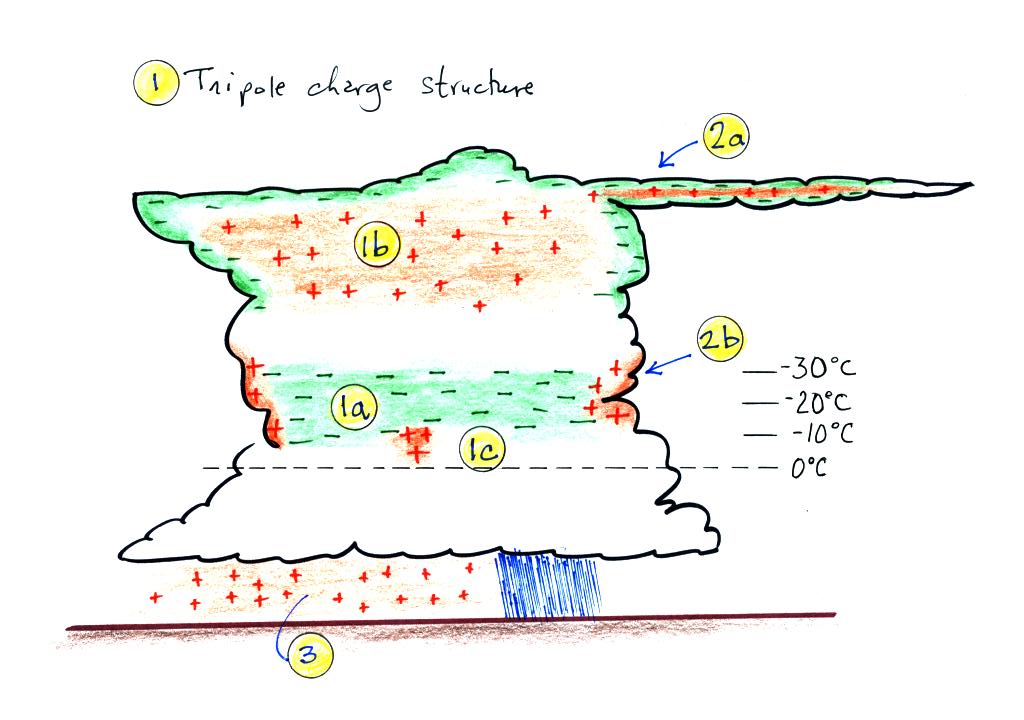
This is the last picture of the day. It shows the normal
distribution of charge in a thunderstorm. This is what a viable
cloud electrification process needs to be able to explain.
Note first of all the cloud has a rough tripolar structure
consisting of a main negative charge center (1a), an upper positive
charge center (1b), and lower positive charge centers (1c). All
are found at temperatures colder than freezing. The main layer of
negative charge (1a) seems always to be found at temperatures between
-10 C and -30 C.
Screening layers are found at the top and sides of the cloud (2a
and 2b in the figure). These form because of the abrupt drop in
conductivity as you move from outside the cloud into the cloud.
E fields under the thunderstorm at the ground are typically 1000s
of V/m (100 to 300 V/m is typically found during fair weather).
Enhancement of the E field at the points of sharp objects on the ground
often go into corona discharge and spray positive charge into the air
near the ground. The ground under the main part of
the thunderstorm is also positively charged.