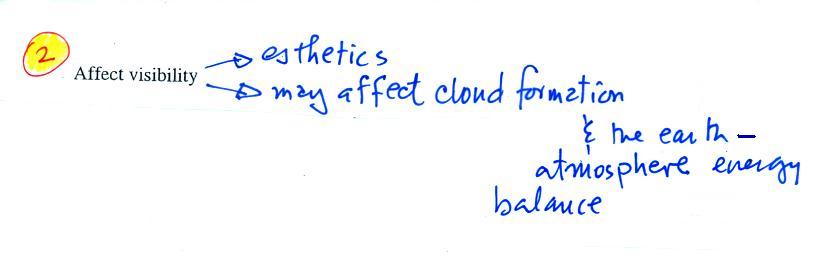
| CO (carbon monoxide) |
O3
(ozone) |
SO2
(sulfur dioxide) |
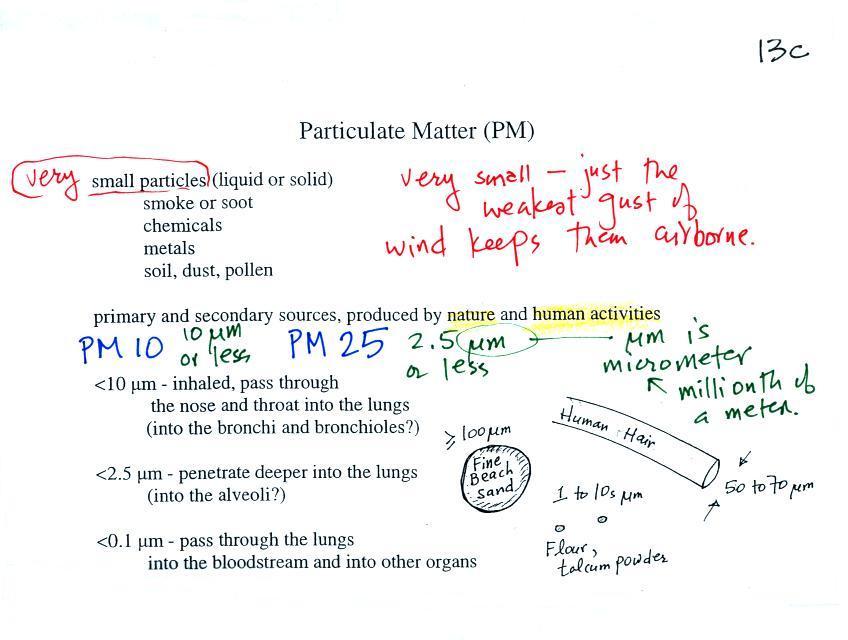
PM (particulate
matter) |
| 1. colorless, odorless, toxic |
1. secondary pollutant |
1. 1st recognized pollutant |
1. Health hazard |
| 2. primary pollutant |
2. summer afternoon |
2. London type smog |
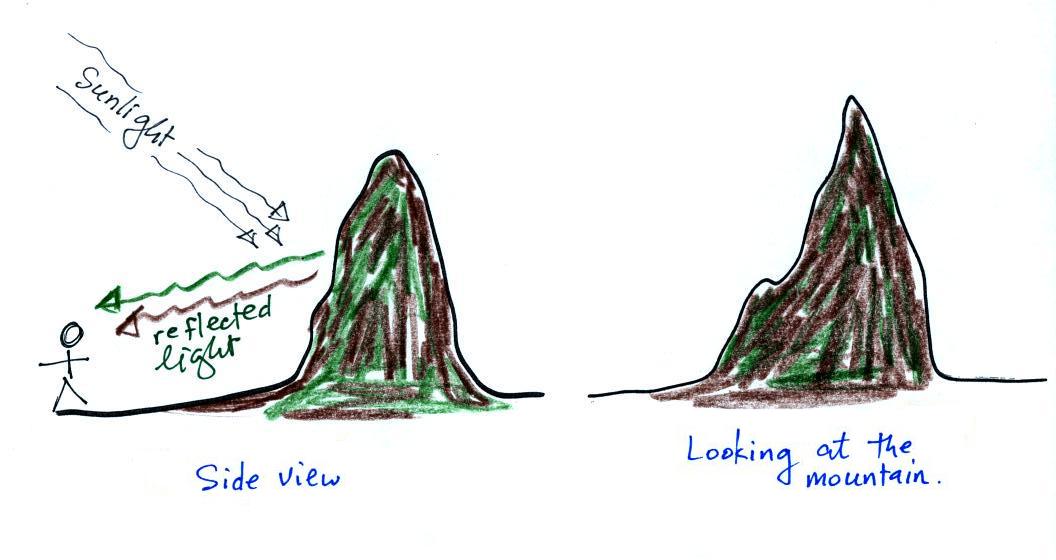
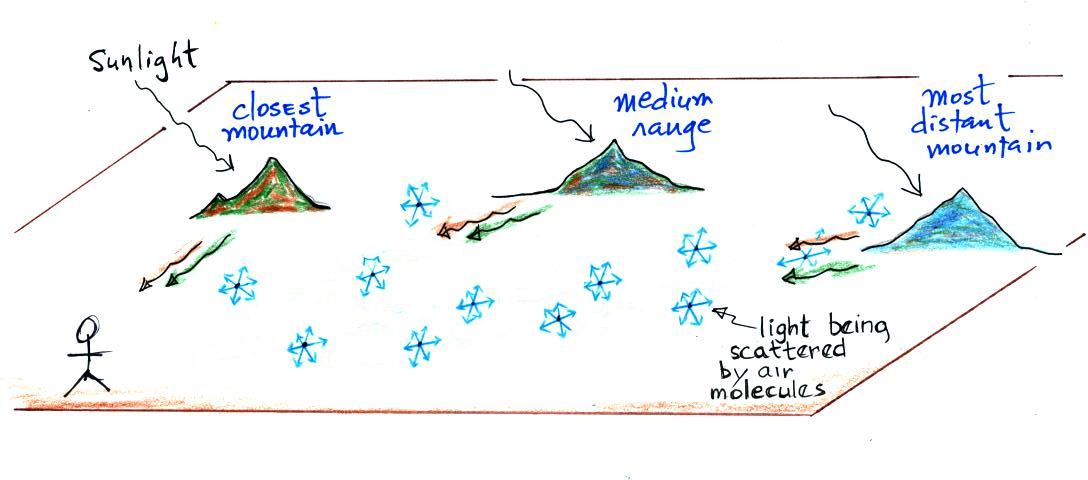
2. Affects visibility |
| 3. incomplete combustion |
3. photochemical (Los Angeles) smog |
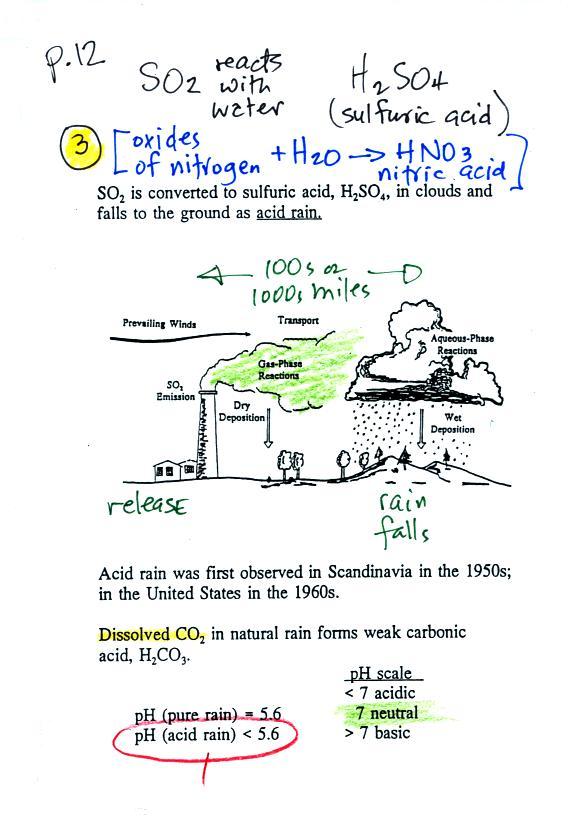
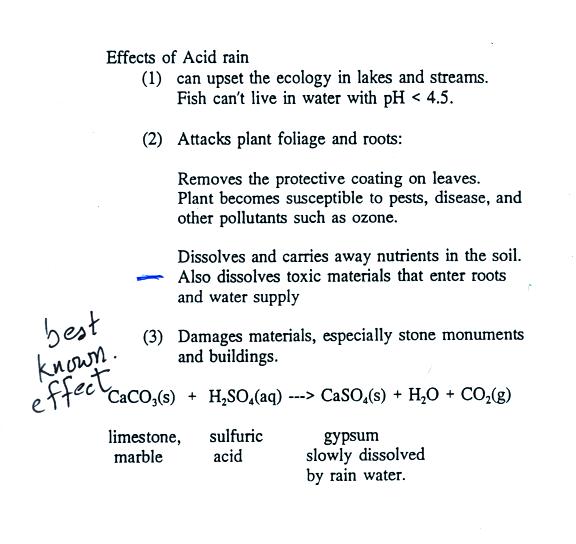
3. Reacts with water in
clouds to make acid rain |
|
| 4. winter morning |
|||
| 5. temperature inversions |


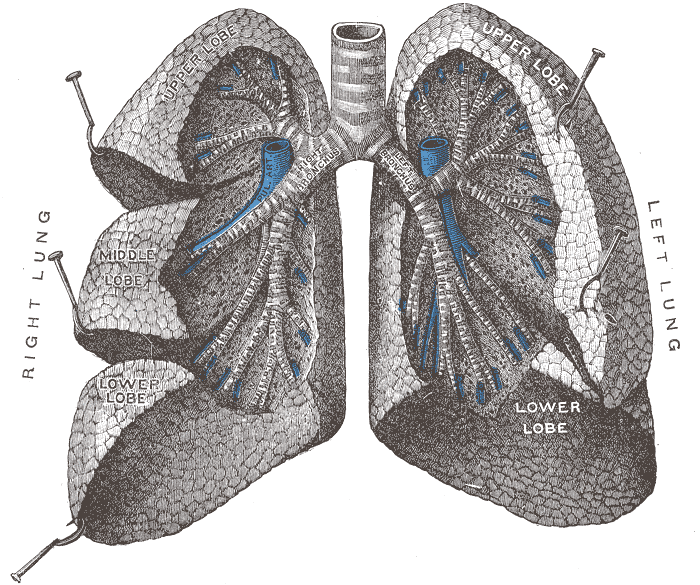 Crossectional view of
the human lungs
from: http://en.wikipedia.org/wiki/Lung |
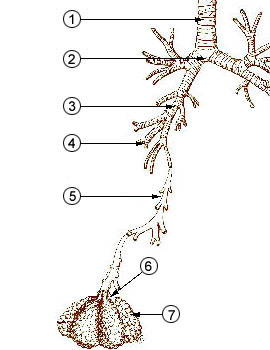 1 - trachea
from
http://en.wikipedia.org/wiki/Image:Illu_quiz_lung05.jpg2 - mainstem bronchus 3 - lobar bronchus 4 - segmental
bronchi
5 - bronchiole6 - alveolar duct 7 - alveolus |