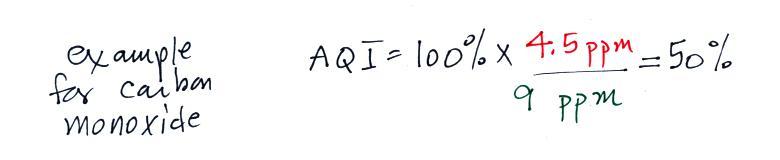Wednesday Jan. 16, 2013
click here to download today's notes
in a more printer friendly format
A new discovery: Lake Street Dive. We only had time to
listen to "You
Go Down Smooth" but you might (or might not) want to check
out "Henriette"and
"Got Me
Fooled" .
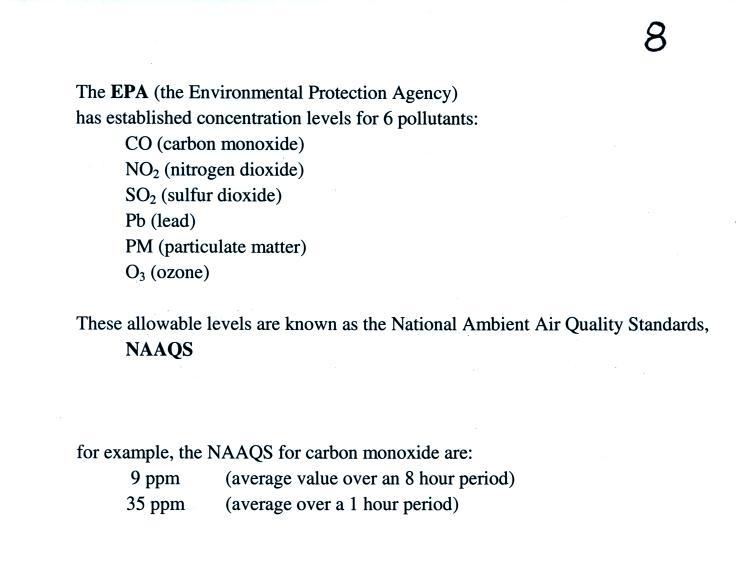
A large city like Tucson is required to continuously monitor
concentrations of several air pollutants. The main ones are
shown below (this is an improved version of information at
the top of p. 8 in the ClassNotes).
The concentration of lead in air has decreased significantly
since lead was removed from gasoline (the following quote is from a Wikipedia article
on gasoline: "In the US,standards to phase out leaded gasoline
were first implemented in 1973 ..... In 1995, leaded fuel accounted
for only 0.6% of total gasoline sales ...... From 1 January 1996,
the Clean Air Act banned the sale of leaded
fuel for use in on-road vehicles. Possession and use of leaded
gasoline in a regular on-road vehicle now carries a maximum $10,000
fine in the US.")
In Tucson, carbon monoxide, ozone, and particulate matter are
of primary concern and daily measurements are reported in the city
newspaper. Let suppose a CO concentration of 4.5 ppm (8 hour
average) was measured yesterday in Tucson. Would this be an
acceptable or hazardous value? Most people wouldn't be able
to answer that question. So rather than report the actual
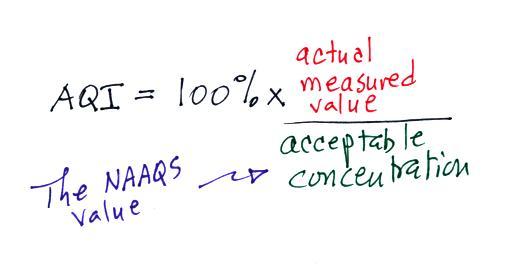
measured values, an Air Quality Index value is reported
instead. The AQI is the ratio of the measured to
accepted concentrations multiplied by 100%.
If we plug in the 3 ppm value
mentioned above for carbon monoxide, the AQI value would be
The air quality in this case would be good. Air becomes
unhealthy when the AQI value exceeds 100%. The
units "ppm", by the way, stand for "parts per million." A CO
concentration of 4.5 ppm would mean that in 1 million air
molecules 4.5 of them would be carbon monoxide.
This information is found on the bottom of p. 8 in the photocopied
ClassNotes. Current
Air Quality Index values for Tucson are available online.
Carbon
monoxide is a serious hazard indoors where is can build
to much higher levels than would ever be found
outdoors. This next link is to a newspaper article
describing an incident at
Virginia Tech (that occurred near the beginning of the
school year in 2007). Carbon
monoxide
from
a malfunctioning hot water heater sickened 23 Virginia
Tech students in an apartment complex. The CO
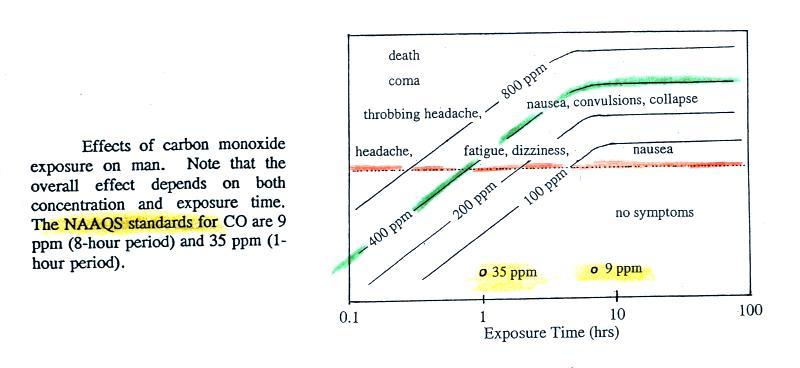
concentration is thought to have reached 500 ppm. You
can get an idea of what kinds of health effects
concentrations this high could cause from the figure. on p.
9 in the photocopied ClassNotes.
You would begin to show symptoms of carbon monoxide exposure
(headache, dizziness, nausea) after breathing a 400 ppm CO
concentrations after about 1 hour. After several hours
exposure you would approach the level where CO would cause coma
and death. At Virginia Tech several students were found
unconscious and one or two had stopped breathing but they were
revived.
Carbon monoxide alarms are relatively inexpensive (~$50) and
are available at most hardware stores. They will monitor CO
concentrations indoors and warn you when concentrations reach
hazardous levels. Indoors CO is produced by gas
furnaces and water heaters that are either operating improperly or
aren't being properly vented to the outdoors. A few hundred
people are killed indoors by carbon monoxide every year in the
United States. An operating carbon monoxide
alarm probably saved the lives of the
6 Tucson residents in December 2010. You can learn
more about carbon monoxide hazards and risk prevention at the Consumer
Product Safety Commission web page.
Next we turned our attention to ozone, another outdoor
pollutant of concern.
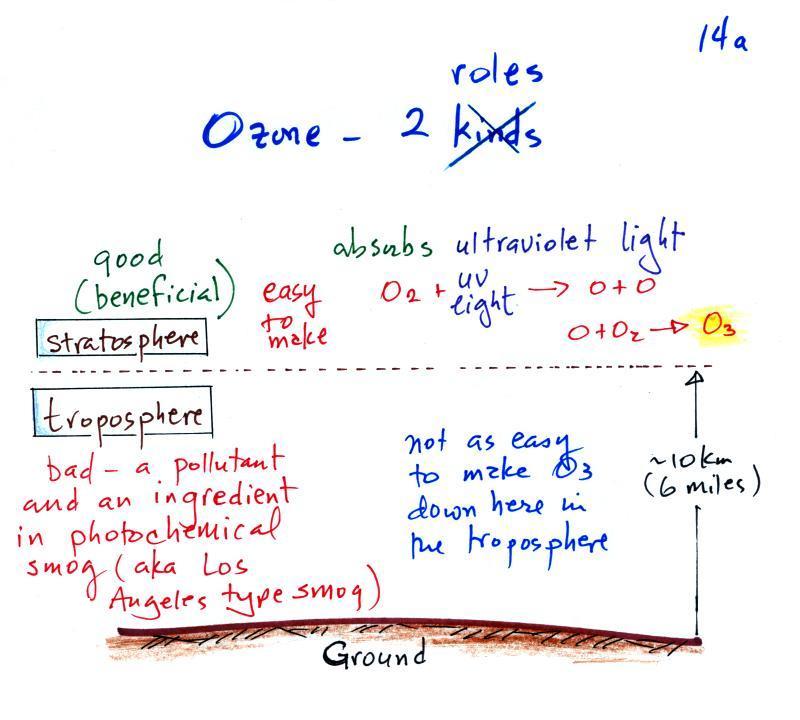
Ozone has a kind of Dr.
Jekyll
and
Mr Hyde personality.
The figure above can be found on p. 14a in the photocopied
ClassNotes. The ozone layer (ozone in the stratosphere) is
beneficial, it absorbs dangerous high energy ultraviolet light
(which would otherwise reach the ground and cause skin cancer,
cataracts, etc. There are some types of UV light that would
quite simply kill us).
Ozone in the troposphere is bad, it is toxic and a
pollutant. Tropospheric ozone is also a key component of
photochemical smog (also known as Los Angeles-type smog)
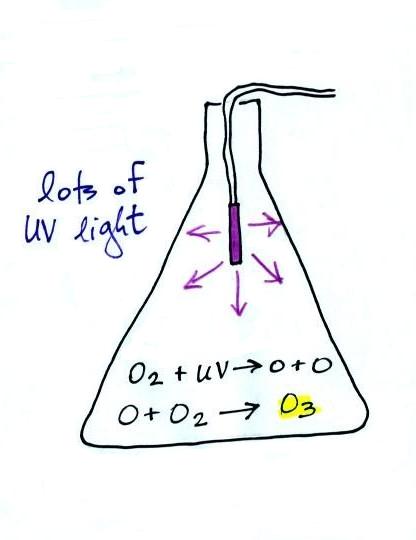
We'll be making some photochemical smog in a class
demonstration. To do this we'll first need some ozone; we'll
make use of the simple stratospheric recipe (shown above) for
making what we need instead of the more complex tropospheric
process (the 4-step process in the figure below). You'll
find more details a little further down in the notes.
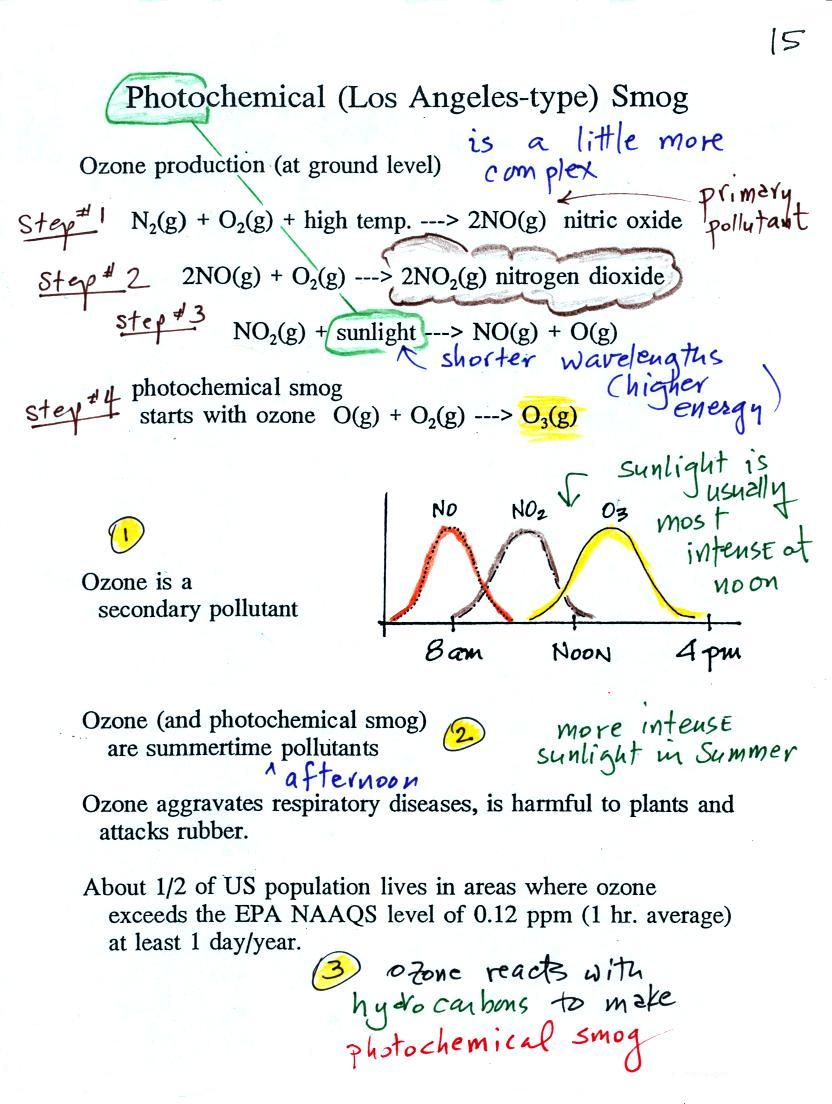
At the top of this figure (p. 15 in the packet of
ClassNotes) you see that a more complex series of reactions is
responsible for the production of tropospheric ozone. The
production of tropospheric ozone begins with nitric oxide
(NO). NO is produced when nitrogen and oxygen in air are
heated (in an automobile engine for example) and react.
The NO can then react with oxygen in the air to make nitrogen
dioxide, the poisonous brown-colored
gas that I've been thinking about making in class (one of
the concerns I have with making nitrogen dioxide in class is
summed up by the following statement from the Wikipedia article: "Symptoms
of poisoning (lung edema) tend to appear
several hours after inhalation of a low but potentially fatal
dose. Also, low concentrations (4 ppm) will anesthetize
the nose, thus creating a potential for overexposure.")
Sunlight can dissociate (split) the nitrogen dioxide molecule
producing atomic oxygen (O) and NO. O and O2 react in a 4th step to make ozone (O3) just like happens in the
stratosphere. Because ozone does not come directly from an
automobile tailpipe or factory chimney, but only shows up after a
series of reactions in the air, it is a secondary
pollutant. Nitric oxide (NO) would be the primary
pollutant in this example.
NO is produced early in the day (during the morning rush
hour). The concentration of NO2 peaks somewhat later. Because sunlight is
needed in step #3 and because sunlight is usually most intense at
noon, the highest ozone concentrations are usually found in the
afternoon. Ozone concentrations are also usually higher in
the summer when the sunlight is most intense.
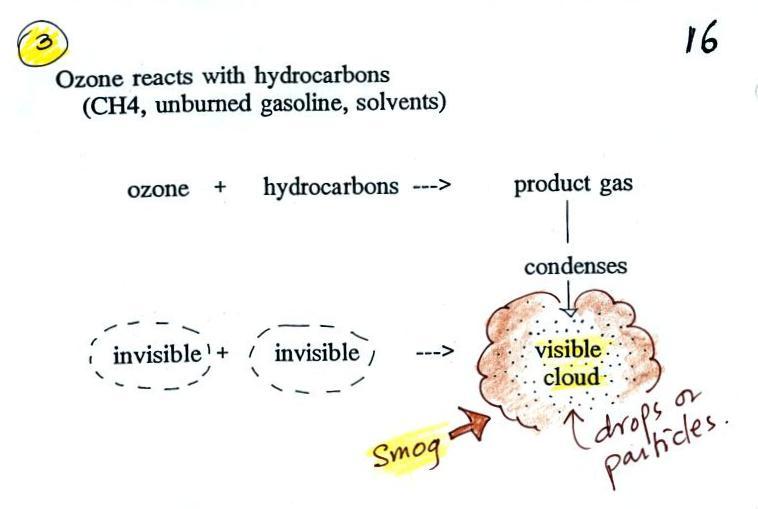
Once ozone is formed, the ozone can react with a hydrocarbon of
some kind to make a product gas. The ozone, hydrocarbon, and
product gas are all invisible, but the product gas sometimes
condenses to make a visible smog cloud or haze. The cloud is
composed of very small droplets or solid particles. They're
too small to be seen but they are able to scatter light - that's
why you can see the cloud.
Here's a pictorial summary of the photochemical smog
demonstration.
We started by putting a small "mercury vapor" lamp inside a
flash. The bulb produces a lot of ultraviolet light (the
bulb produced a dim bluish light that we could see, but the UV
light is invisible so we had no way of really telling how bright
it was). The UV light and oxygen in the air produced a lot
of ozone (you could easily have smelled it if you had taken the
cover off the flask).
After a few minutes we turned off the lamp and put a few pieces
of lemon peel into the flash. Part of the smell that comes
from lemon peel is limonene, a hydrocarbon. The limonene gas
reacted with the ozone to produce a product gas of some
kind. The product gas condensed, producing a visible smog
cloud (the cloud was white, not brown as shown above). I
meant (but forgot) to shine the laser beam through the smog
cloud to reinforce the idea that we are seeing the cloud because
the drops or particles scatter light.
We had a little extra time and moved onto to the 3rd pollutant
that we will consider, sulfur dioxide. But first here's a a
list of the main points for the two pollutants that we've covered
so far. This figure wasn't shown
in class.
The third pollutant we'll cover is sulfur dioxide.
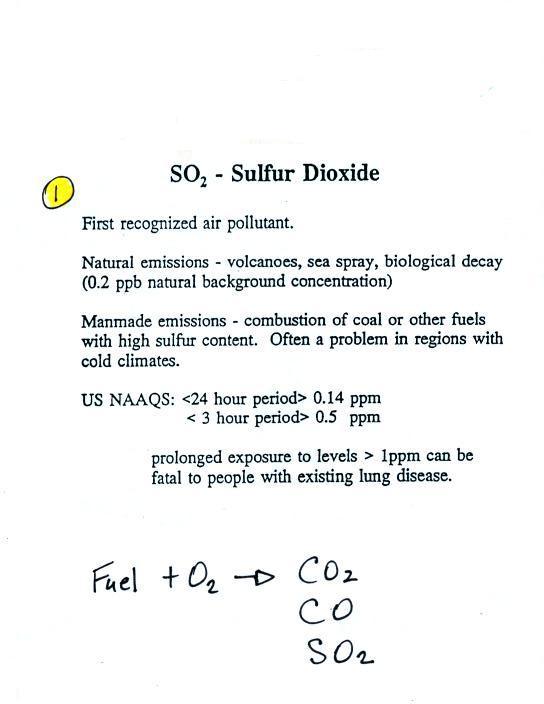
Sulfur dioxide is produced by
the combustion of sulfur containing fuels such as coal.
Combustion of fuel also produces carbon dioxide and carbon
monoxide. People probably first became aware of sulfur
dioxide because it has an unpleasant smell (described as the
smell of "rotten eggs"). Carbon dioxide and
carbon monoxide are odorless. That is most likely why
sulfur dioxide was the first pollutant people became aware of.
Volcanoes are a natural source of sulfur dioxide.
Some of the photographs below come from articles published in
2002 on the 50th anniversary of the event.
The sulfur dioxide didn't kill people directly.
Rather it would aggravate an existing condition of some
kind. The SO2
probably also made people susceptible to bacterial infections
such as pneumonia. Here's
a link that
discusses
the event and its health effects in more detail.
Some other air pollution disasters also involved high SO2 concentrations. One of the deadliest
events in the US occurred in 1948 in Donora, Pennsylvania.
"This eerie photograph was taken at noon on Oct. 29,
1948 in Donora, PA as deadly smog enveloped the town. 20 people
were asphyxiated and more than 7,000 became seriously ill during
this horrible event."
The photograph below shows some of the mills that were
operating in Donora at the time. The factories were not
only emitted pollutants into the air but probably also
discharging pollutants into the river.
from: http://oceanservice.noaa.gov/education/kits/pollution/02history.html
from: http://www.eoearth.org/article/Donora,_Pennsylvania
"When Smoke Ran Like
Water," a book about air pollution is among the books that you
can check out, read, and report on to fulfill part of the
writing requirements in this class (though I would encourage you
to do an experiment instead). The author, Devra Davis,
lived in Donora Pennsylvania at the time of the 1948 air
pollution episode. Another book that I've just learned
about "Killer Smog: The World's
Worst Air Pollution Disaster" by William Wise is an account of the
London Smog of 1952 (I don't yet have a copy of that book)
There's one more thing to learn about sulfur dioxide, but we'll
have to wait until Friday to do that.