Wednesday Feb. 27, 2013
click here to download today's notes
in a more printer friendly format
Keb'Mo' sung "Better Man"
before class this afternoon.
After a long delay I am happy to be able to say that the last of the 1S1P Assignment
#1 reports (Carbon Dioxide) have been graded. Several
students turned in a third report even though there was a two
report limit for this assignment. I read those reports but
didn't record the grades. Nonetheless you should hang on to
your report because you may have an opportunity later in the
semester to turn it in for credit.
Now that 1S1P Assignment #1 is in the book Assignment #2 is starting to
take shape. Two topics are available online and I plan to
add a third soon. Reports on the first two topics aren't due
until after Spring Break.
The Experiment #1 revised reports were collected today.
Materials for Experiment #3 will be
distributed in class on Friday.
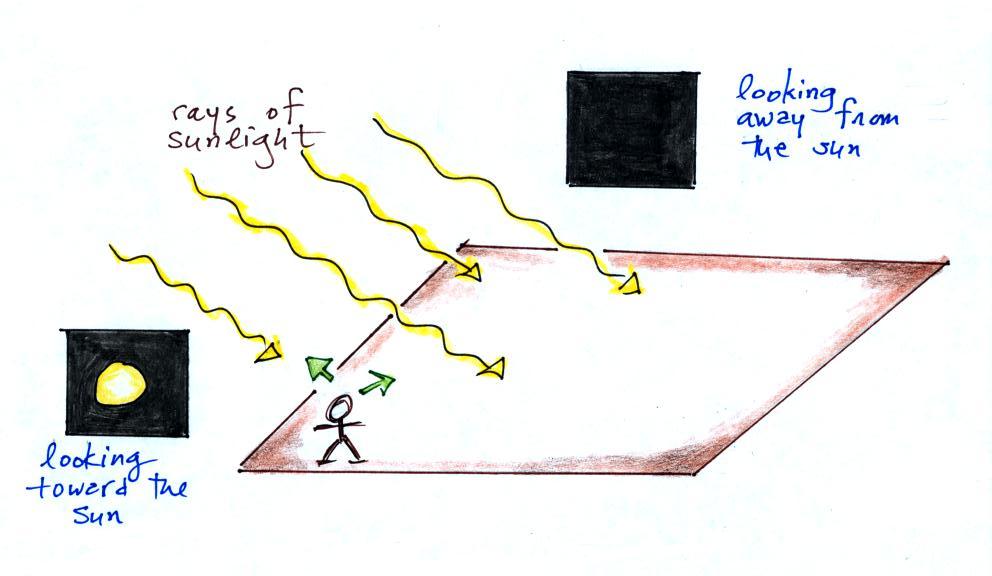
We were talking about filters in class on Monday and a
student asked whether filtering of sunlight is what causes the sky
to appear blue. The blue color of the sky is caused by
scattering of sunlight. The following series of pictures
tries to explain how this works (this discussion also appears at
the end of the Mon., Jan. 14 online
class notes)
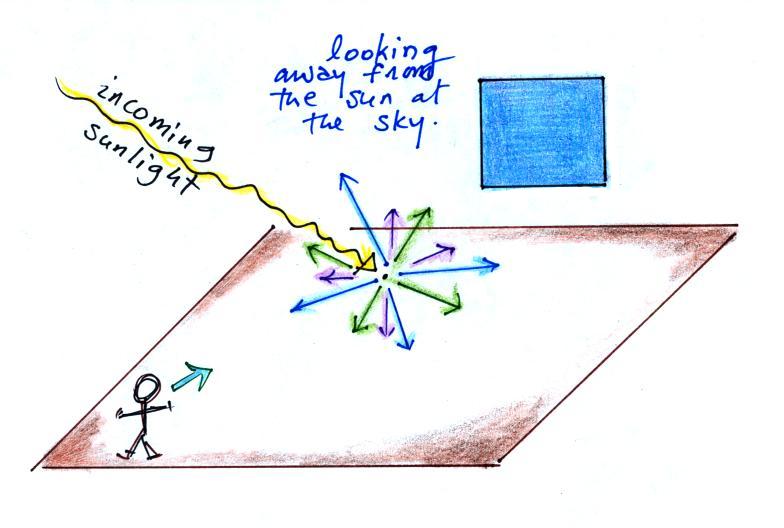
You can find all kinds of things in the sky: air,
particulates, clouds, etc. But first let's
imagine there isn't an atmosphere. No air, clouds,
particulates, nothing.
If you went outside and looked
at the sun (you shouldn't do that of course) you'd see a
bright white sun against a black background. You'd
see the sun because you're looking back in the direction
of one of the rays of light coming from the sun.
If you look away from the sun and
toward the sky you wouldn't see anything. The
sky would appear black. That's because there's
nothing to scatter the sunlight. This is just
like when you couldn't see the laser beam as it
traveled across the front of the classroom (in the
light scattering demonstration early in the
semester). You couldn't see the beam unless
something was put into the beam to scatter some of the
laser light.
In
the
next
picture
we'll
add an atmosphere. Just air molecules, no
particles, or clouds.
Air molecules
scatter light. We didn't see this light in
the laser demonstration because the laser light
scattered by the air was too weak. But when
you're dealing with intense sunlight traveling
through a lot more air in the atmosphere you can
see the scattered light.
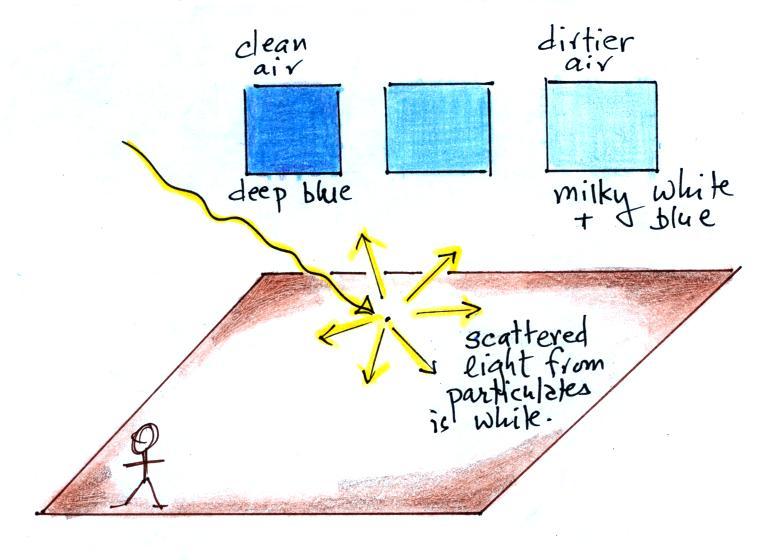
The incoming sunlight is white. White light
is a mixture of all the colors. Air
molecules scatter the shorter wavelengths (violet
blue green) more than the longer wavelengths
(yellow orange red). This is depicted
above. Air molecules scatter light in this
way because they are very small (much smaller than
the wavelength of visible light).
Violet has the shortest wavelength and is
scattered the most. However there isn't as
much violet in sunlight as there is blue and
green. There's a lot of green light in
sunlight (more than any other color as a matter of
fact) but it isn't scattered as readily as
blue. So the end result is that we see blue
light coming from the sky. This is why the
sky is blue. When the air is clean (from of
particulates), the sky has a deep blue
color. Here's
a
little more explanation of why the mixture
of violet, blue, and green appears blue.
Next we'll add a cloud to the picture. As we
saw in the laser demonstration, cloud droplets and
ice crystals are good scatters of light.
Cloud droplets and ice crystals though are much
larger than air molecules. Because of this
they scatter all the colors in equal amounts.
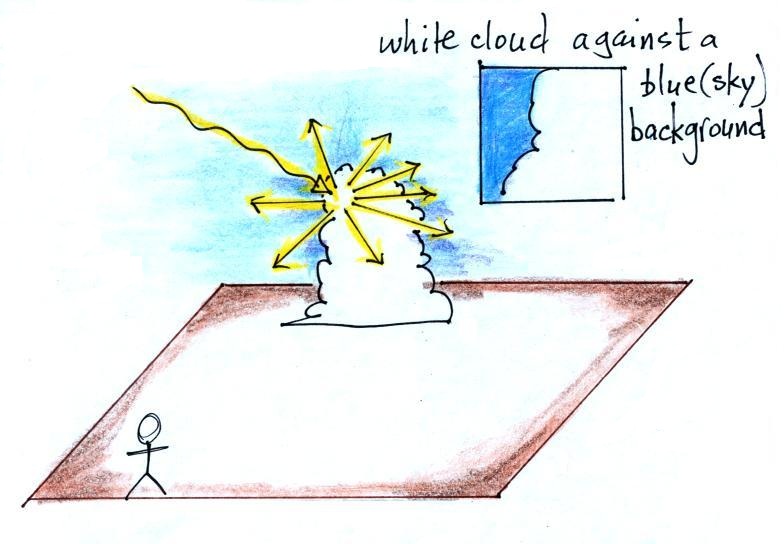
When white
light strikes a cloud, white light is scattered
and reflected. This is why clouds are white
(with some shades of grey mixed in if the cloud is
thick). When you look up at a cloud you see
a white cloud (sunlight being scattered by cloud
droplets) surrounded by blue sky (sunlight being
scattered by air molecules.
What about particles? Particulates are much
bigger than air molecules and a little bit smaller
than cloud droplets. They scatter light is
the same way that cloud droplets and ice crystals
do. The scattered light from particles is
white.
What do you see now when you look at
the sky? It depends on how much particulate matter
is in the air. When the air is clean and doesn't
contain much particulate matter the sky is a deep
blue. As the concentration of particulates increases
you mix in more and more white light. The color of
the sky can change to a whitish blue when the particulate
concentration is high.
The world would not look the same if we were able to see IR
light instead of visible light.

|

|
visible light
reflected by the tree
and photographed with normal
film
|
near IR light
reflected by the tree
and photographed using IR
film
|
The picture at left was taken using normal film, film that is
sensitive to visible light. The picture at right used
infrared film. In both pictures we are looking at sunlight
that strikes the tree or the ground and is reflected
toward the camera where it can be photographed (i.e. these aren't
photographs of light emitted by the tree or the ground). The
tree at left is green and relatively dark (it reflects green light
but absorbs the other colors of visible light). The tree at
right and the ground are white, almost like they were covered with
snow. The tree and grass on the ground are reflecting
infrared light. Here are many
more images taken with infrared film.

Here's another example, photographs of the ground taken from an
air plane using ordinary film at left (responds to visible light)
and infrared film at right. Notice how the IR
photograph is able to "see through" the haze. The haze
at left is scattered light. IR light is not scattered as
readily as visible light.
Another example was shown in class, a thermal
image of a house. These are photographs of
infrared light that is being emitted (not reflected light) by a
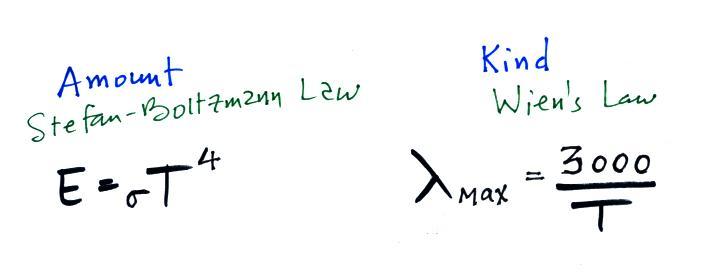
house. Remember that the amount of energy emitted by an
object depends strongly on temperature (temperature to the 4th
power in the Stefan-Boltzmann law). Thus it is possible to
see hot spots that emit a lot of energy and appear "bright" and
colds spots. Photographs like these are often used to
perform an "energy audit" on a home, i.e. to find spots where
energy is being lost. Once you locate one of these hot spots
you can add insulation and reduce the energy loss. Don't
worry too much about the colors. The photograph is probably
taken using just a single wavelength. The thing that varies
is the intensity of the IR light. Processing of the
photograph adds color to make differences in intensity more
apparent. Reds and orange mean more intense emission of IR
radiation (warmer temperature) than the blues and greens.
We'll something similar when we look at IR satellite photographs
of clouds.
One last point which might not have been
mentioned in class: the pictures of reflected
IR light are probably looking at near IR light with a wavelength
of 1 μm (light with a wavelength just a little bit longer than
visible light). The photographs of emitted IR light at
capturing far IR emissions, light with a wavelength of 10 μm.
We now have most of the tools we will need to begin to study
radiant energy balance on the earth. It will be a balance
between incoming sunlight energy and outgoing IR radiation emitted
by the earth. This will ultimately lead us to an explanation
of the atmospheric greenhouse effect.
We will first look at the simplest kind of situation, the earth
without an atmosphere (or at least an atmosphere without
greenhouse gases). The next figure is on p. 68 in the
photocopied Classnotes. Radiative equilibrium is really just
balance between incoming and outgoing radiant energy.
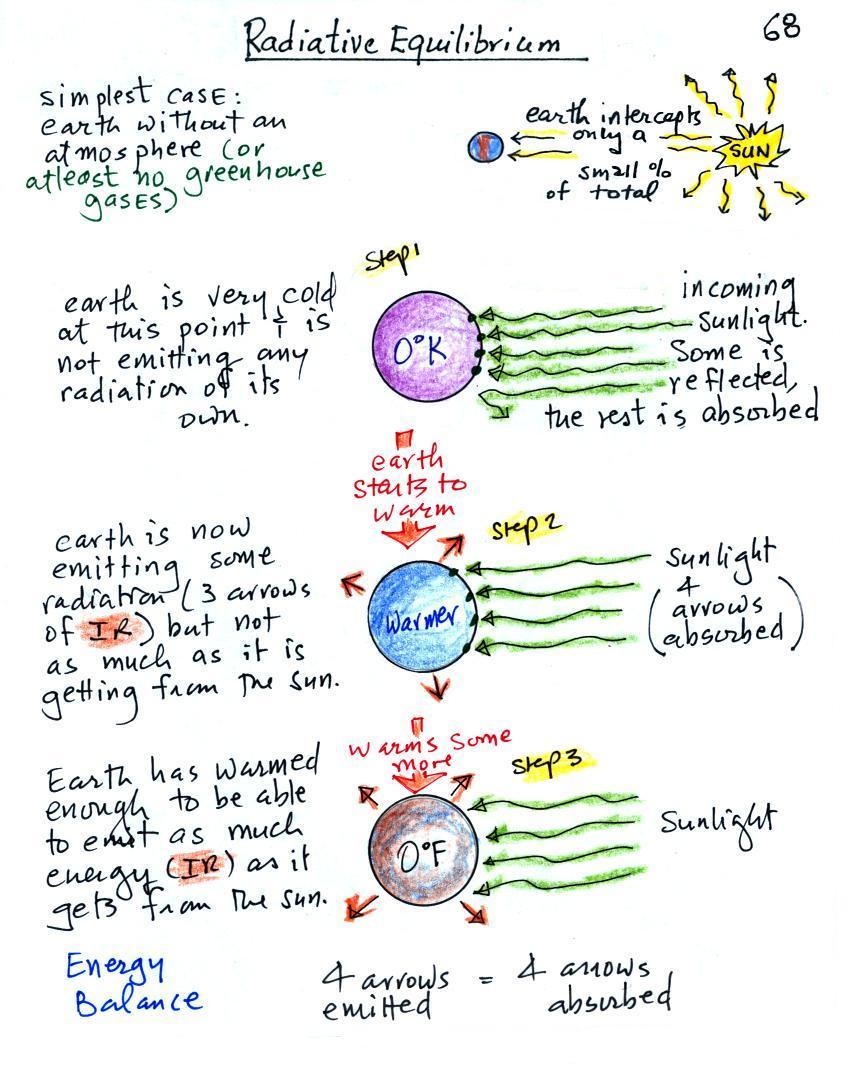
You might first wonder how it is possible for the earth (with a
temperature of around 300 K) to be in energy balance with the sun
(6000 K). The sun is bigger, hotter, and emits much more
radiant energy than the earth. At the top right of the
figure you can see that because the earth is located about 90
million miles from the sun and it only absorbs a very small
fraction of the total energy emitted by the sun. The earth
only needs to balance the energy is absorbs from the sun.
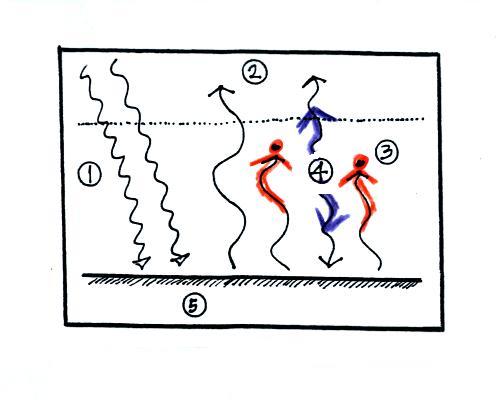
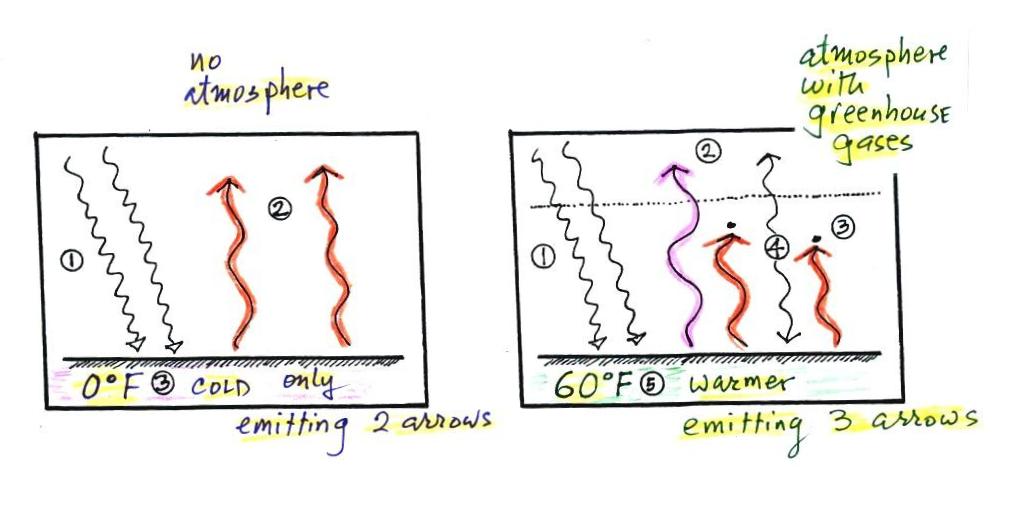
To understand how energy balance occurs we start, in Step #1,
by imagining that the earth starts out very cold (0 K) and is not
emitting any EM radiation at all. It is absorbing sunlight
however (4 of the 5 arrows of incoming sunlight in the
first picture are absorbed, 1 of the arrows is being reflected) so
it will begin to warm This is like opening a bank account,
the balance will start at zero. But then you start making
deposits and the balance starts to grow.
Once the earth starts to warm it will also begin to emit EM
radiation, though not as much as it is getting from the sun (the
slightly warmer earth in the middle picture is now colored
blue). Only the four arrows of incoming sunlight that are
absorbed are shown in the middle figure. The arrow of
reflected sunlight has been left off because they don't really
play a role in energy balance (reflected sunlight is
like a check that bounces - it really doesn't affect your bank
account balance). The earth is emitting 3 arrows
of IR light (in red). Because the earth is still
gaining more energy (4 arrows) than it is losing (3 arrows) the
earth will warm some more. Once you find money in
your bank account you start to spend it. But as long as
deposits are greater than the withdrawals the balance will grow.
Eventually it will warm enough that the earth (now shaded brown
& blue) will emit the same amount of energy as it absorbs from
the sun. This is radiative equilibrium, energy balance (4
arrows of absorbed energy are balanced by 4 arrows of emitted
energy). The temperature at which this occurs is about 0
F. That is called the temperature of radiative equilibrium
(it's about 0 F for the earth).
Note that it is the amounts of energy not the kinds of energy
that are important. Emitted radiation may have a different
wavelength than the absorbed energy. That doesn't
matter. As long as the amounts are energy the earth will be
in energy balance. Someone might deposit money into your
bank account in Euros while you spend dollars.
Before we start to look at radiant energy balance on the
earth with an atmosphere we need to learn about how the atmosphere
will affect the incoming sunlight and outgoing IR light emitted by
the earth. We'll draw a filter absorption graph for the
earth's atmosphere.
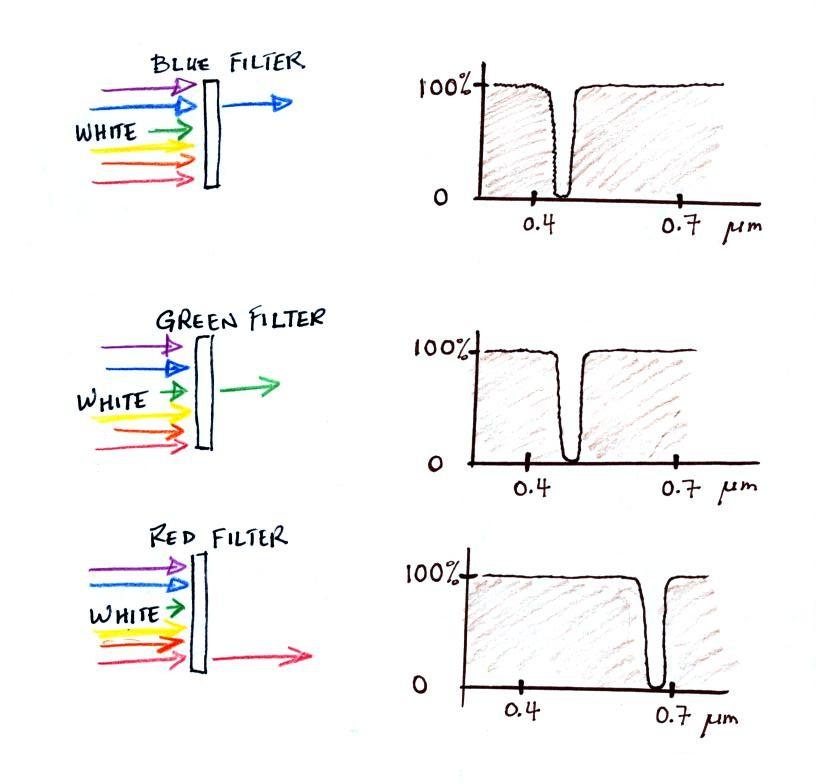
We will first look at the effects simple blue, green,
and red glass filters have on visible light. This is just to
be sure we understand what an absorption curve
represents.
If you try to shine white light (a mixture of all the colors)
through a blue filter, only the blue light passes through.
The filter absorption curve shows 100% absorption at all but a
narrow range of wavelengths that correspond to blue light.
The location of the slot or gap in the absorption curve shifts a
little bit with the green and red filters.
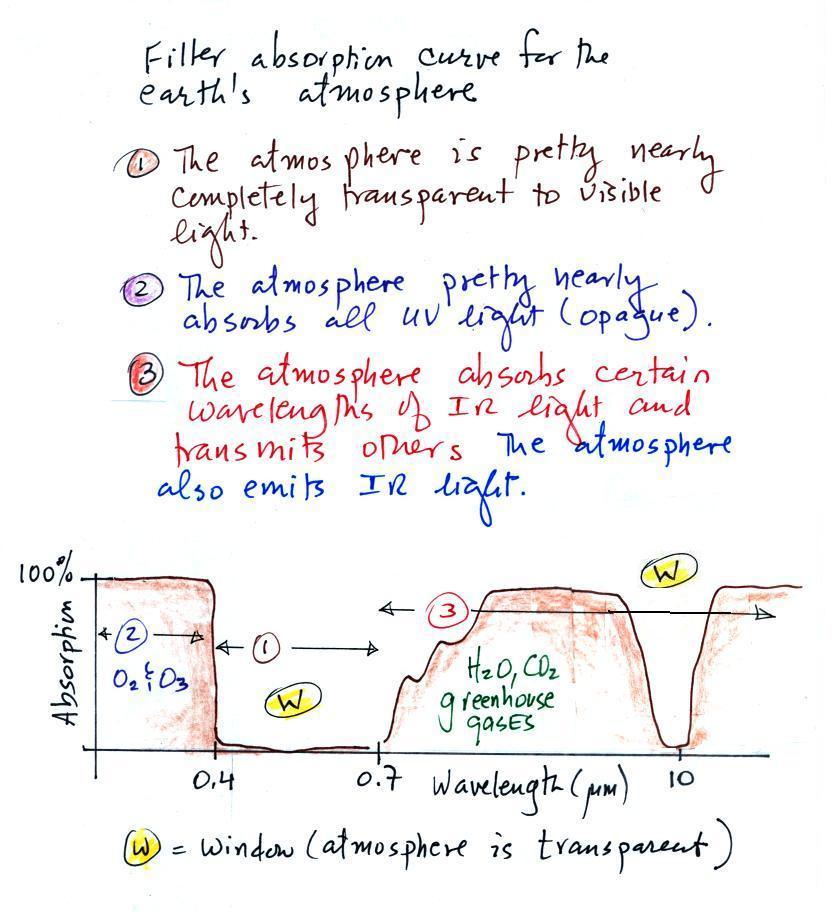
The following figure is a simplified, easier to remember,
representation of the filtering effect of the atmosphere on UV,
VIS, and IR light (found on p. 69 in the photocopied notes).
The figure was redrawn after class.
You can use your own eyes to tell you what effect the
atmosphere has on visible light. Air is clear, it is
transparent. The atmosphere transmits visible light.
In our simplified representation oxygen and ozone make the
atmosphere pretty nearly completely opaque to UV light (opaque is
the opposite of transparent and means that light is blocked or
absorbed; light can't pass through an opaque material). We
assume that the atmosphere absorbs all incoming UV light, none of
it makes it to the ground. This is of course not entirely
realistic.
Greenhouse gases make the atmosphere a selective absorber of IR
light - the air absorbs certain IR wavelengths and transmits
others . Wavelengths between 0.7 and
8 or 9 μm are absorbed,
radiation centered at 10μm is
transmitted by the atmosphere. Wavelengths greater than 10 μm
are absorbed (again by greenhouse gases). It is the
atmosphere's ability to absorb certain wavelengths of infrared
light that produces the greenhouse effect and warms the surface of
the earth. The atmosphere also emits IR radiation.
This is also an important part of the greenhouse effect.
Note "The atmospheric window" centered at 10 micrometers. Light emitted by the
earth at this wavelength (and remember 10 um is the wavelength of
peak emission for the earth) will pass through the
atmosphere. Another transparent region, another window, is
found in the visible part of the spectrum.
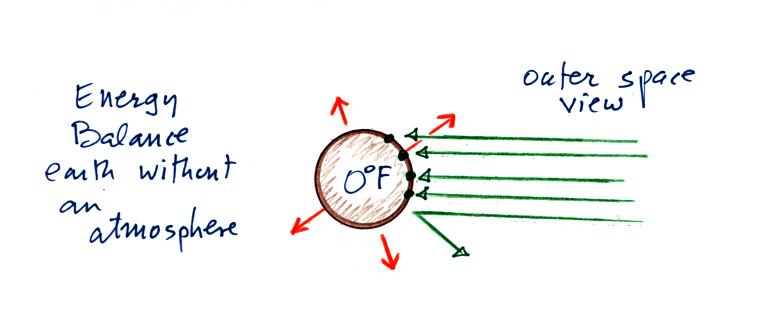
Now back to the outer space view of radiative equilibrium on
the earth without an atmosphere. The important thing to note
is that the earth is absorbing and emitting the same amount of
energy (4 arrows absorbed balanced by 4 arrows emitted). The
arrow of reflected sunlight doesn't any role at all.
We will be moving from outer space to the earth's surface (the
next two figures below).
Don't let the fact that there are
4 arrows are
being absorbed and emitted in the figure above and
2 arrows absorbed and emitted in the bottom figure below
bother you. The important
thing is that there are equal amounts being absorbed and emitted
in both cases.
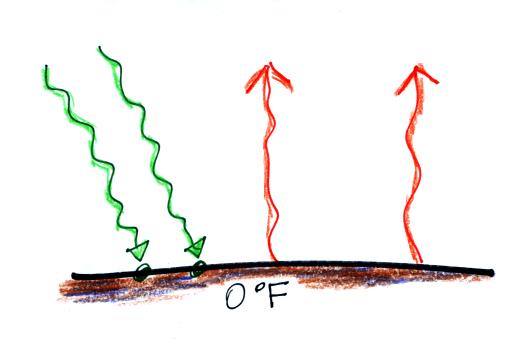
The reason for only using two
arrows in this picture is to keep the picture as simple as
possible. It will get complicated enough when we add the
atmosphere to the picture.
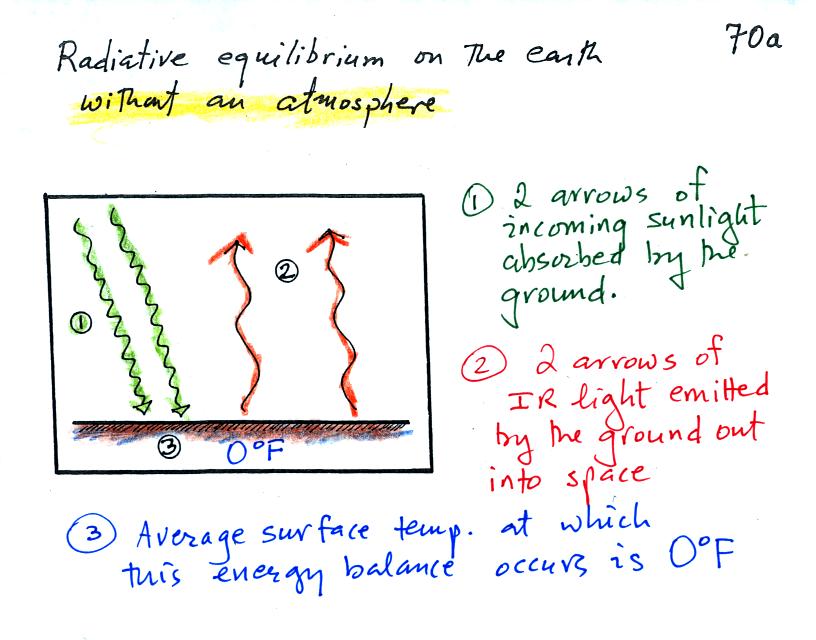
Here's the same picture with
some more information added (p. 70a in the photocopied
ClassNotes). This represents energy balance on the earth
without an atmosphere.
The next step is to add the atmosphere.
We will study a simplified
version of radiative equilibrium just so you can
identify and understand the various parts of the picture.
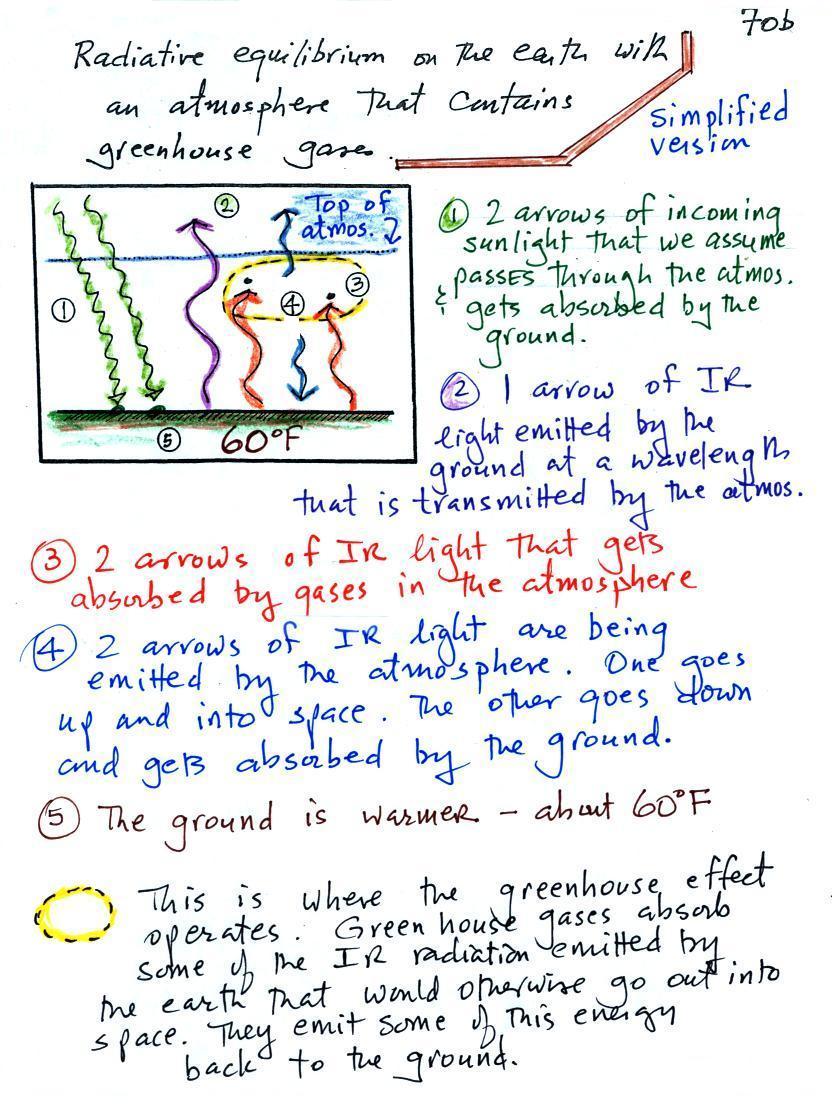
Keep an eye out for the greenhouse effect. Here's a
cleaned up version of what we ended up with in class (I added a
little information at the bottom of the picture.
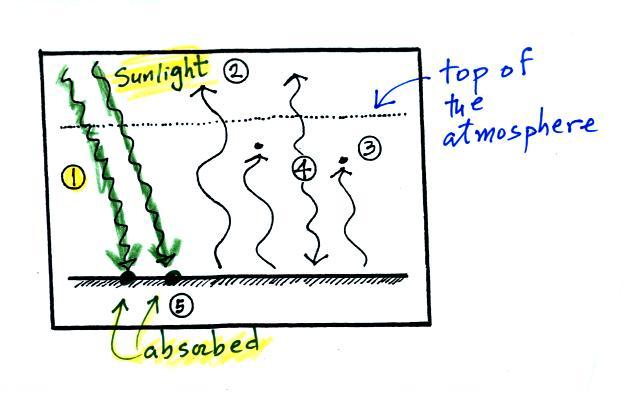
It would be hard to sort through
and try to understand all of this if you weren't in class
(difficult enough even if you were in class). So below we
will go through it again step by step (which you are free to
skip over if you wish). This is a more detailed version than
was done in class. Caution: some of the colors
below are different from those used in class.
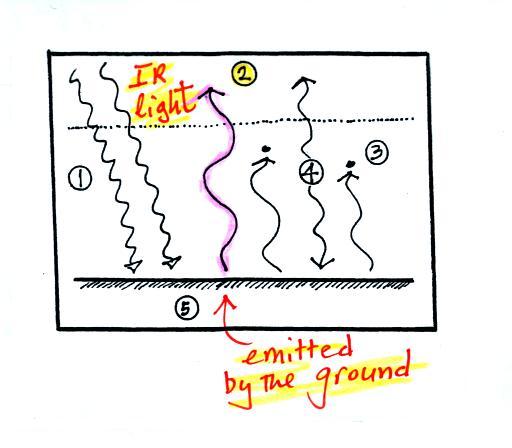
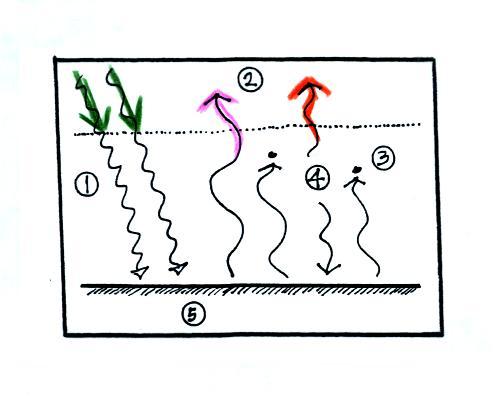
1. In
this picture we see the two rays of incoming sunlight that
pass through the atmosphere, reach the ground, and are
absorbed. 100% of the incoming sunlight is transmitted
by the atmosphere. This wouldn't be too bad of an
assumption if sunlight were just visible light. But it
is not, sunlight is about half IR light and some of that is
going to be absorbed. But we won't worry about that at
this point.
The ground is
emitting a total of 3 arrows of IR radiation. At this
point that might seem like a problem. How can the
earth emit 3 arrows when it is absorbing only 2. We'll
see how this can happen in a second.
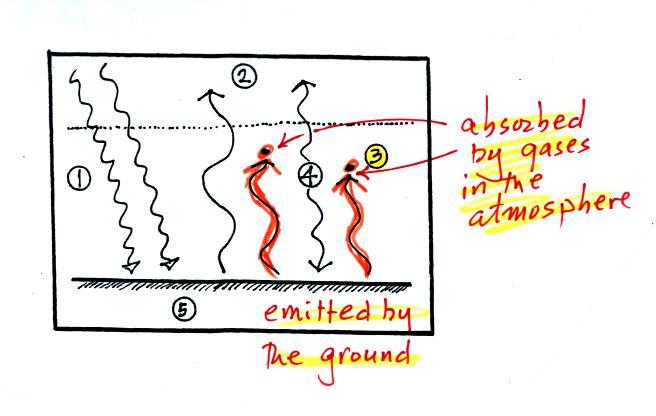
2. One
of
these
(the
pink
or
purple
arrow
above)
is
emitted
by
the
ground
at
a
wavelength
that
is
not absorbed
by greenhouse gases in the atmosphere (probably around 10
micrometers, in the center of the "atmospheric
window"). This radiation passes through the atmosphere
and goes out into space.
3. The other 2
units of IR radiation emitted by the ground are absorbed by
greenhouse gases is the atmosphere.
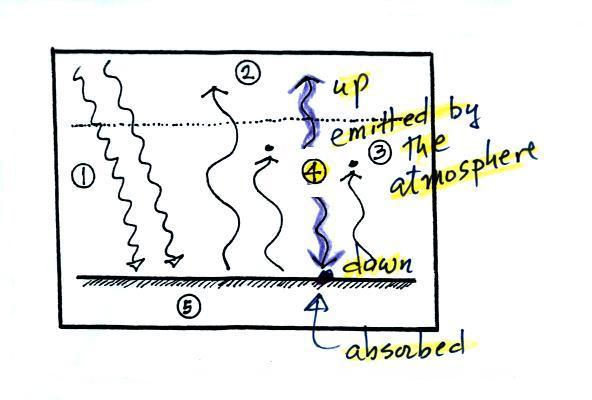
4. The atmosphere is absorbing 2 units of
radiation. In
order to be in radiative equilibrium, the atmosphere must
also emit 2 units of radiation. That's shown
above. 1 unit of IR radiation is sent upward into
space, 1 unit is sent downward to the ground where it is
absorbed. This is probably the part of the picture
that most students have trouble visualizing (it isn't so
much that they have trouble understanding that the
atmosphere emits radiation but that 1 arrow is emitted
upward and another is emitted downward toward the ground.
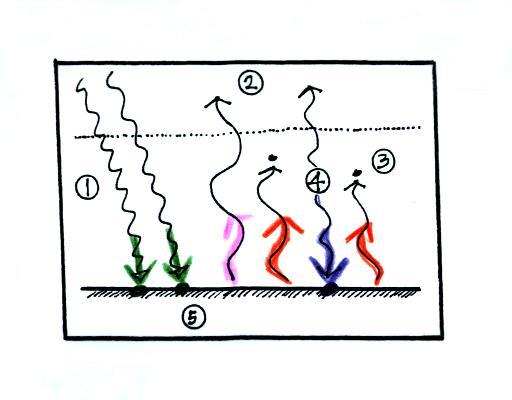
Before we go any further we will
check to be sure that every part of this picture is in energy
balance, something we didn't have time to do in class.
The ground is
absorbing 3 units of energy (2 green arrows of sunlight and
one blue arrow coming from the atmosphere) and emitting 3
units of energy (one pink and two red arrows). So the
ground is in energy balance.
The atmosphere is
absorbing 2 units of energy (the 2 red arrows coming from
the ground) and emitting 2 units of energy (the 2 blue
arrows). One goes upward into space. The
downward arrow goes all the way to the ground where it gets
absorbed (it leaves the atmosphere and gets absorbed by the
ground). The atmosphere is in energy balance.
And we should check
to be sure equal amounts of energy are arriving at and
leaving the earth. 2 units of energy arrive at the top
of the atmosphere (green) from the sun after traveling
through space, 2 units of energy (pink and orange) leave the
earth and head back out into space. Energy balance
here too.
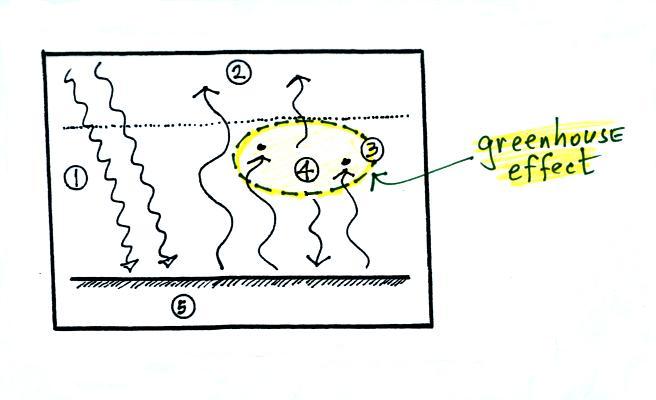
The greenhouse
effect involves the absorption and emission of IR radiation
by the atmosphere. Here's how you might put it into
words
Doesn't it make sense that if the ground is getting back some of
the energy it would otherwise lose, the ground will end up being
warmer. That's what the greenhouse effect does, it warms the
earth's surface. The global annual average surface
temperature is about 60 F on the earth with a greenhouse
effect. It would be about 0 F without the greenhouse effect.
Here are a couple other ways of understanding why the
greenhouse effect warms the earth. We
didn't cover this material in class but I'll stick
it in here anyway just to complete this part of the topic.
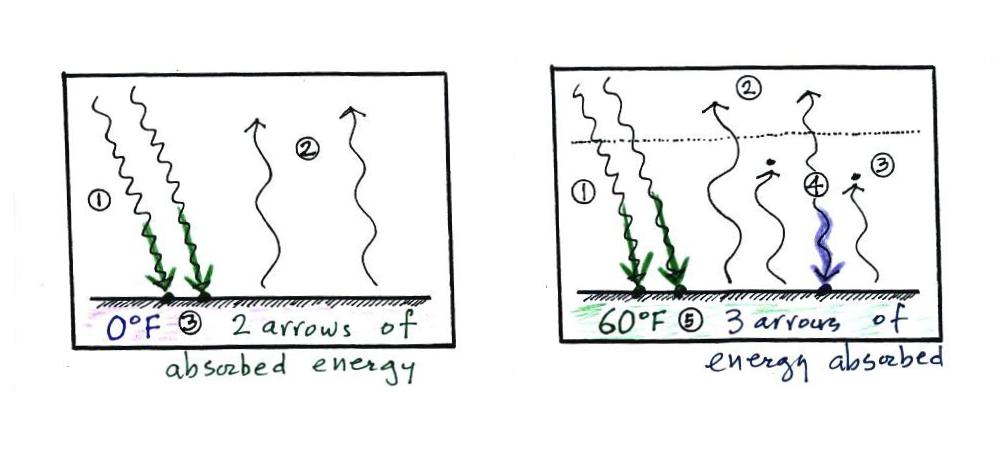
The picture at left
is the earth without an atmosphere (without a greenhouse
effect). At right the earth has an atmosphere, one
that contains greenhouse gases. At left the ground is
getting 2 units of energy (from the sun). At right it
is getting three, two from the sun and one from the
atmosphere (thanks to the greenhouse effect). Doesn't
it seem reasonable that ground that absorbs 3 units of
energy will be warmer than ground that is only absorbing 2?
Here's another, more subtle, explanation of why the ground
is warmer with a greenhouse effect than without.
At left the ground only needs to emit 2 units of
energy to be in energy balance, at right the ground must emit
3 units to be in balance. Remember that the amount of
energy emitted by something depends on temperature (the left
equation below).
The cold ground in the left picture above must warm in order
to be able to emit 3 arrows of energy needed in the right
picture.