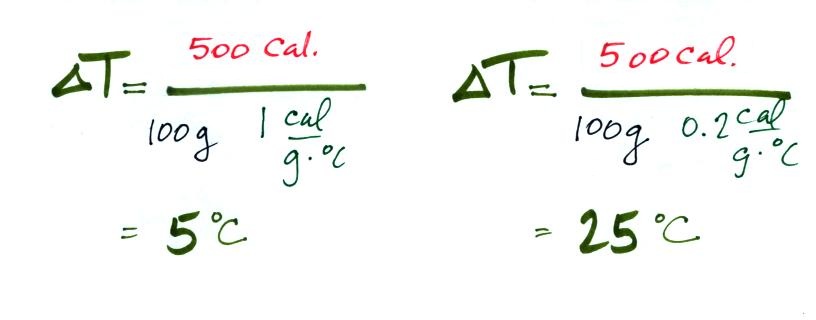Friday Feb. 15, 2013
click here to download today's notes
in a more printer friendly format
Calexico Quattro
(live at the Barbican Theater in London).
Calexico is one of my favorite local groups. They'll
probably be back for an encore performance later this semester.
Here's a
good video of the meteorite that hit somewhere in Russia
earlier today. It caused some damage and injured several
hundred people apparently. And a second
video with sound of the explosion. After
reading some more about the meteorite, it sounds like the
explosion was mostly just the shock wave produced as the meteorite
fell through the atmosphere rather than the sound of impact.
Much of the meteorite probably burned up in the atmosphere, though
some fragments did reach the ground.
Quiz #1 has been graded and was returned in class today.
There were some very high grades and also many very low
grades. I suspect that many students aren't putting in the
effort that is needed to do well on the quizzes
(such as reading the online notes when they appear after
class). Please come see me by during
office hours if you did poorly on the quiz and are working hard at
trying to understand and learn the course material. In many
cases it isn't a question of not devoting enough time but studying
the material in the right kind of way.
An "in-class Optional Assignment" was handed out in class
today. The idea is to work on the assignment in class and
turn it in at the end of the period. If you weren't in class
and would like to do the assignment you can download a
copy here and turn in the completed assignment at the
beginning of class next Monday. You'll receive at least
partial credit.
OK we're done with weather maps for the time being. Though
if interesting weather appears imminent I'll try to mention it in
class.
If we were using a textbook in this class we'd be moving into
Chapter 2! During the next couple of weeks we
will be concerned with energy, temperature, heat, energy
transport, and energy balance between the earth, atmosphere, and
space.
It is easy to lose sight of the main concepts because there
are so many details. The following is an introduction to
this new section of material and most of the figures are found
on pages 43 & 44 in the photocopied ClassNotes.
Types of energy
We will learn the names of
several different types or forms of energy.
Kinetic energy is energy of motion. Some examples (both
large and microscopic scale) are mentioned and sketched
above. This is a relatively easy to visualize and
understand form of energy.
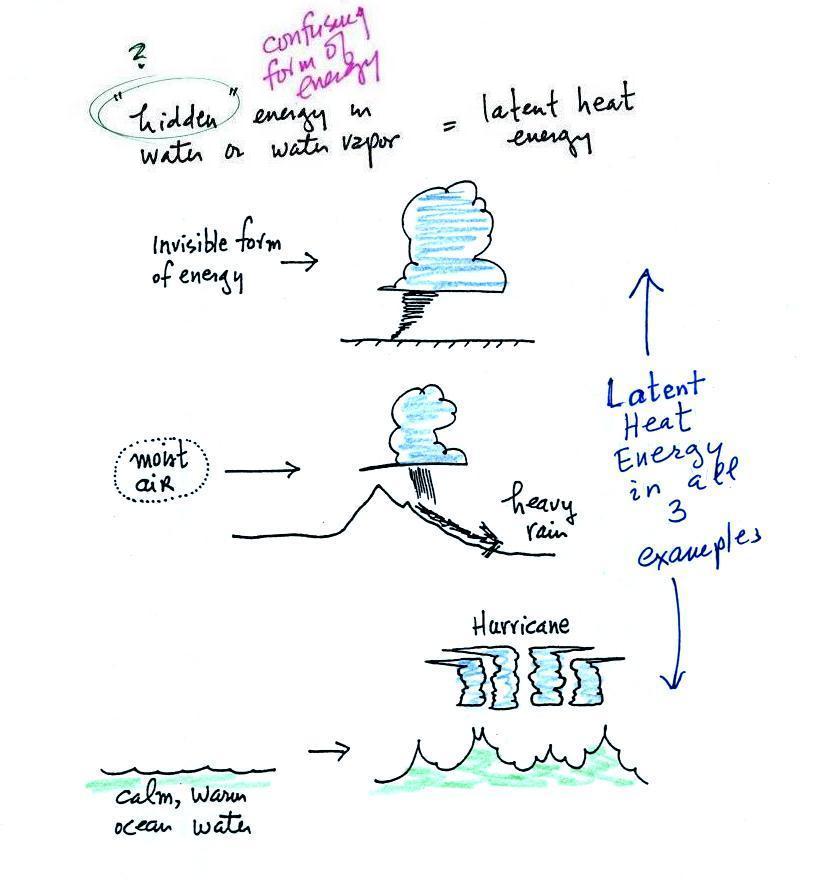
Latent heat energy is an under-appreciated and rather
confusing type of energy. The word latent refers to energy that
is hidden in water and water vapor. The hidden energy
emerges when water vapor condenses or water freezes (the energy
had been added earlier when ice was melted or water was
evaporated). The fact that the energy is hidden is part of
what makes it confusing.
Radiant energy is a very important form of energy that was
for some reason left off the original list in the
ClassNotes. Sunlight is an example of radiant energy that
we can see and feel (you feel warm when you stand in
sunlight). There are many types of radiant energy that are
invisible (such as the infrared light that people emit).
Electromagnetic
radiation is another name for radiant energy.
Energy transport
Four energy transport processes are listed below
By far the most important
process is at the bottom of the list above. Energy
transport in the form of electromagnetic radiation (sunlight for
example) is the only process that can transport energy through
empty space. Electromagnetic radiation travels both to the
earth (from the sun) and away from the earth back into
space. Electromagnetic radiation is also responsible for
about 80% of the energy transported between the
ground and atmosphere.
You might be surprised to learn that latent heat is the
second most important transport process.
Rising parcels of warm air and sinking parcels of cold air
are examples of free convection. Because of convection you
feel colder or a cold windy day than on a cold calm day (the
wind chill effect). Ocean currents are also an
example of convection. Ocean currents transport energy
from the warm tropics to colder polar regions.
Convection is a 3rd way of causing rising air motions in the
atmosphere (convergence into centers of low pressure and fronts
are two other ways we've encountered so far)
Conduction is the least important energy transport at least
in the atmosphere. Air is such a poor conductor of energy
that it is generally considered to be an insulator.
Energy balance and the atmospheric greenhouse effect
The next picture (the figure in
the ClassNotes has been split into three parts for improved
clarity) shows energy being transported from the sun to the
earth in the form of electromagnetic radiation.
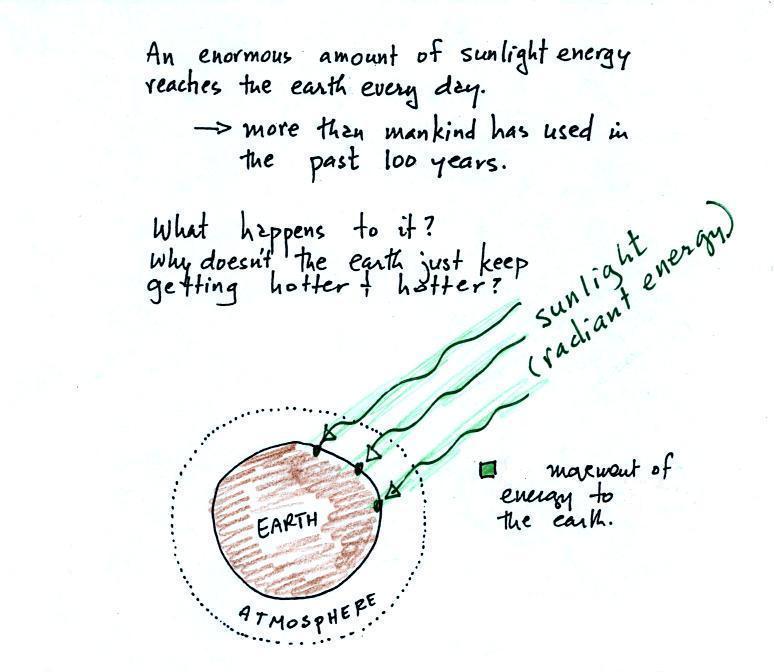
We are aware of this energy
because we can see it (sunlight also contains invisible
forms of light) and feel it. With all of this energy
arriving at and being absorbed by the earth, what keeps the
earth from getting hotter and hotter? If you park your
car in the sun it will heat up. But there is a limit
to how hot it will get. Why is that?
It might be helpful when talking about energy balance to
think of a bank account. If you periodically deposit
money into your account why doesn't the balance just grow
without limit. The answer is that you also take money
out of the account and spend it. The same is true of
energy and the earth. The earth absorbs incoming
sunlight energy but also emits energy back into space (the
orange and pink arrows in the figure below). Energy is
being emitted by both the surface of the earth and the
atmosphere.
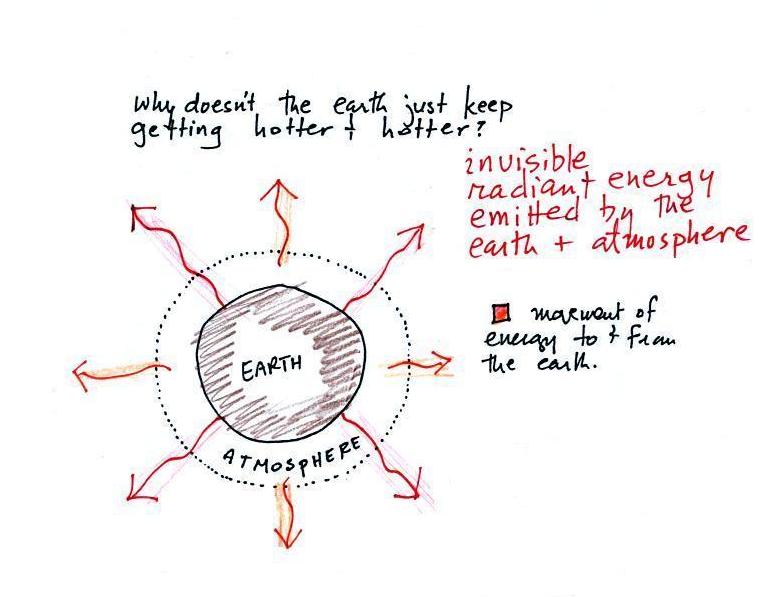
Energy emitted in the form of
infrared light is an invisible form of energy (it is weak enough
that we don't usually feel it either). A balance between
incoming and outgoing energy is achieved and the earth's annual
average temperature remains constant.
We will also look closely at energy transport between the
earth's surface and the atmosphere (see the figure below). This
is where latent heat energy transport, convection and conduction
operate (they can't transport energy beyond the atmosphere and
into outer space).
That is also where the atmospheric greenhouse
functions. That will be a important goal - to better
understand how the atmospheric greenhouse effect works.

The greenhouse effect is getting a lot of "bad press". If
the earth's atmosphere didn't contain greenhouse gases and if
there weren't a greenhouse effect, the global annual average
surface temperature would be about 0 F (scratch out -4 F and put 0
F, it's easier to remember). Greenhouse gases raise this
average to about 60 F and make the earth a much more habitable
place. That is the beneficial side of the greenhouse effect.
The detrimental side is that atmospheric greenhouse gas
concentrations are increasing (no real debate about that).
This might enhance or strengthen the greenhouse effect and cause
the earth to warm (some debate here particularly about how
much warming there might be). While that doesn't
necessarily sound bad it could have many unpleasant side effects
(lots of debate and uncertainty about this also). That's a
subject we'll explore briefly later in the semester.
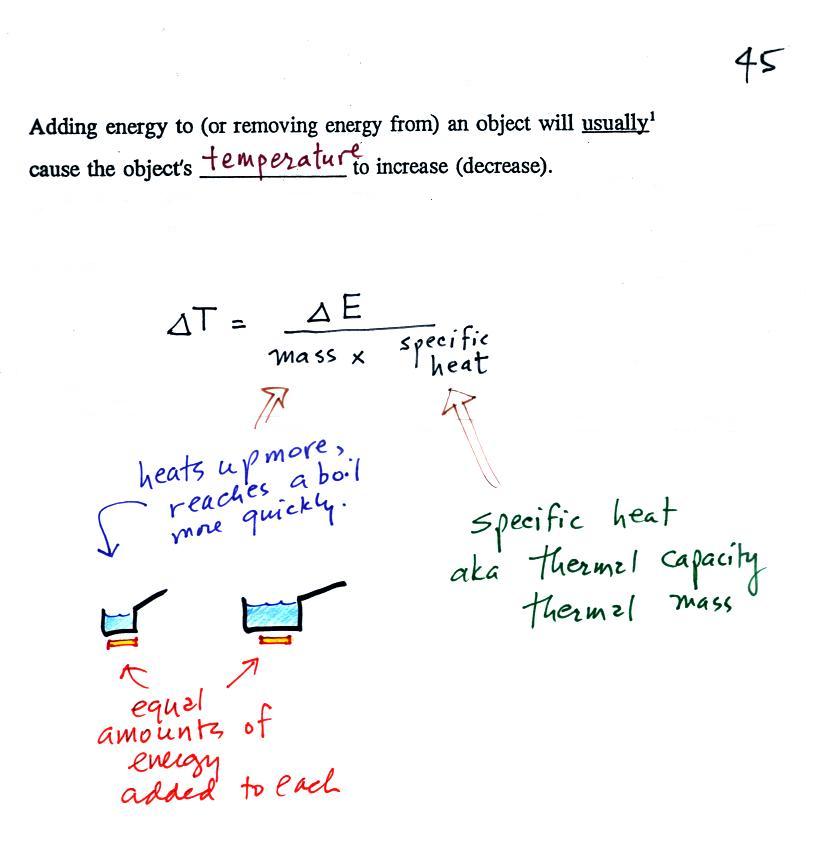
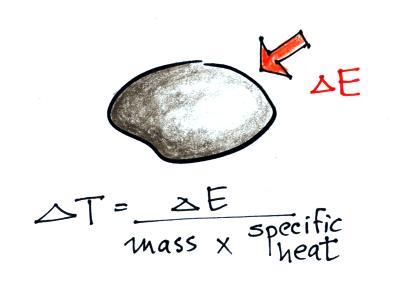
When you add energy to an object, the object will usually warm
up (or if you take energy from an object the object will
cool). It is relatively easy to come up with an equation
that allows you to figure out what the temperature change will be
(this is another equation I'll write on the board before the
next quiz if you ask me to - try to understand it, you don't have
to memorize it).
The temperature change, ΔT, will first depend on how much
energy was added, ΔE. This is a
direct proportionality, so ΔE is in the
numerator of the equation (ΔE and ΔT
are both positive when energy is added, negative when energy is
removed)
When you add equal amounts of energy to large and small
pans of water, the small pan will heat up more quickly. The
temperature change, ΔT, will depend on the
amount of water, the mass. A small mass will mean a large ΔT,
so mass should go in the denominator of the equation.
Specific heat is what we use to account for the fact that
different materials react differently when energy is added to
them. A material with a large specific heat will warm more
slowly than a material with a small specific heat. Specific
heat has the same kind of effect on ΔT as
mass. Specific heat is sometimes called "thermal mass" or
"thermal capacity." You can think of specific
heat as being thermal inertia - a substance with high specific
heat, lots of thermal inertia, will be reluctant to change
temperature.
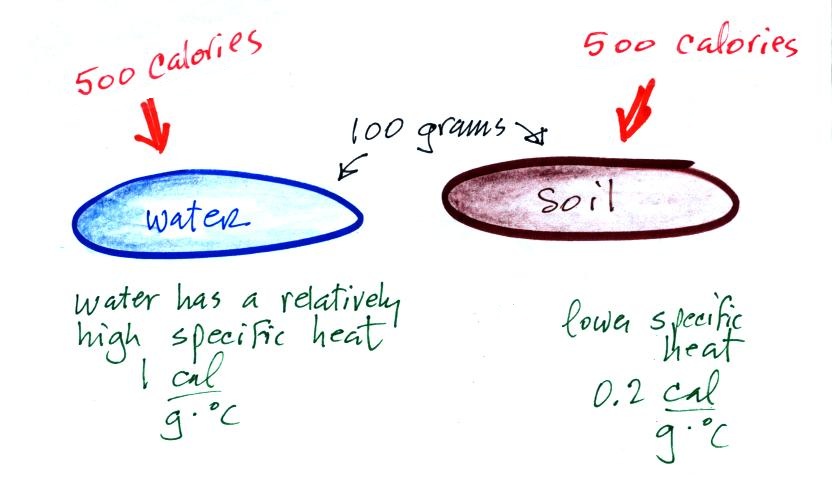
Here's an important example that will show the effect of
specific heat (middle of p. 45).
Equal amounts of energy (500 calories, note that
calories are units of energy) are added to equal masses (100
grams) of water and soil. We use water and soil in the
example because most of the earth's surface is either ocean or
land. Before we do the calculation, try to guess which material
will warm up the most. Everything is the same except for the
specific heats. Will water with its 5 times larger specific
heat warm up more or less than the soil?
The details of the calculation are shown below.
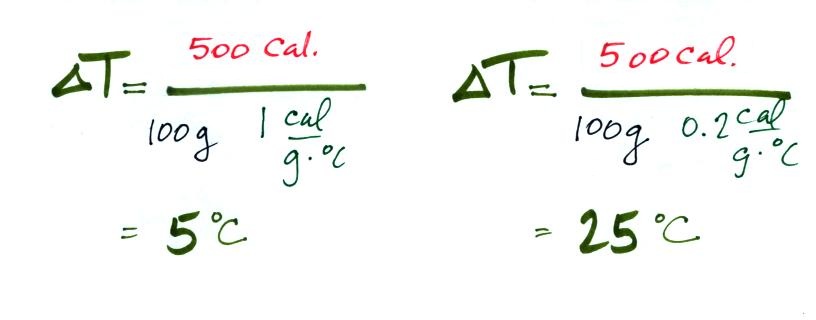
With its higher specific heat, the water
doesn't heat up nearly as much as the soil. If we had
been removing energy the wouldn't cool off as much as the soil
would.
These different rates of warming of water and soil have
important effects on regional climate.
Oceans moderate the climate. Cities near a large body of
water won't warm as much in the summer and won't cool as much
during the winter compared to a city that is surrounded by land.
Water's ΔT is smaller than land's because water has
higher specific heat.
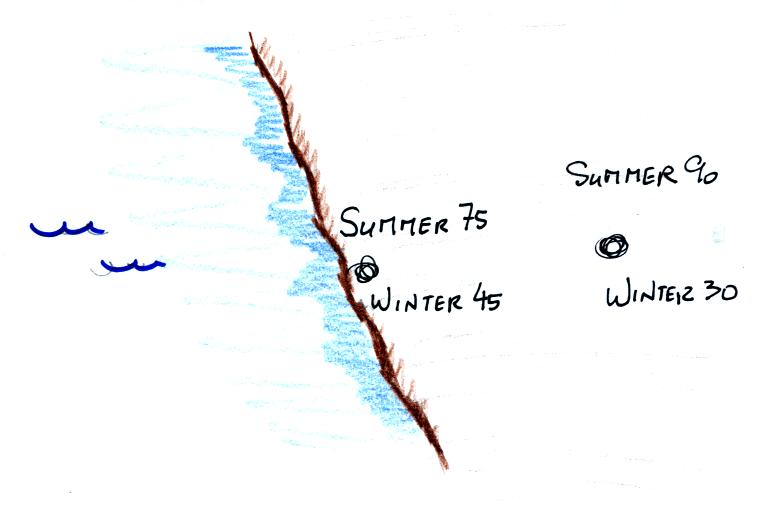
The yearly high and low monthly average temperatures are shown
at two locations above. The city on the coast has a 30o
F annual range of temperature (range is the difference between the
summer and winter temperatures). The city further inland
(assumed to be at the same latitude and altitude) has an annual
range of 60o F. Note that both cities have the
same 60o F annual average temperature. We'll see
a much more dramatic example of the moderating effect of water on
climate in a couple of weeks.
Here's another situation where
you can take advantage of water's high specific heat to
moderate climate on a smaller scale.

It's going to be warm this
weekend and I am going to plant the rest of my tomatoes. I
do this so that they can start to make tomatoes before it starts
to get too hot in May. In February it can still
get cold enough to kill tomatoes so they need some protection (the
broccoli and lettuce in the background can handle a light frost).
Here's one way of doing that. You can surround
each plant with a "wall o water" - a teepee like
arrangement that surrounds each plant. The cylinders are
filled with water and they take advantage of the high
specific heat of water and won't cool as much as the air or soil
would during a cold night. The walls of water produce a warm
moist micro climate that the tomato seedlings love. The
plastic is transparent so plenty of sunlight can get through.
Adding energy to an object
will usually cause it to warm. But there is another
possibility (bottom p. 45), the object could change
phase or state (change from solid to liquid or gas).
Adding energy to ice might cause the ice to melt. Adding
energy to water could cause it to evaporate.

The equation at the bottom of
the figure above allows you to calculate how much energy is
required to melt ice or evaporate water or sublimate dry
ice. You multiply the mass by the latent heat, a variable
that depends on the particular material that is changing
phase. The latent heat of vaporization (evaporation) is
the energy required to evaporate 1 gram of a material.
If you add energy to or remove
energy from an object, the object will usually change
temperature. You can calculate the temperature change if
you know the object's mass and its specific heat. That's
the equation we used in the example calculation above.
It's shown again below.
We (actually you) will conduct an experiment in class next
Monday and will try to measure the energy needed to evaporate
liquid nitrogen. We'll need a way of measuring energy.
If you know the mass and
specific heat of an object and measure a change in temperature
you can use the rearranged equation in the figure above to
calculate how much energy was added to or removed from the
object.