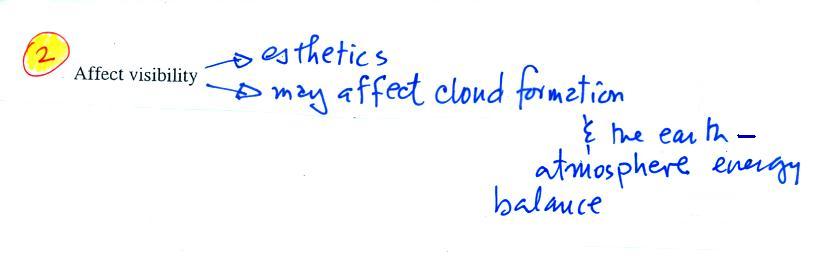Tuesday, Jan. 24, 2012
click here to
download today's notes in a more printer friendly format
Some
gypsy
jazz
(Caravan
at the
Django Reinhardt "Live at Birdland" Festival in New York in 2004) to
get things rolling this week.
Something new in class today, an Optional
In-class Assignment (you work on the assignment and turn it in at
the end of class). If you weren't in class and would like to do
the assignment and earn a little extra credit, you can download the
assignment and turn it in at the start of class on Thursday.
Also the first 1S1P Assignment
is now online. You can write 0, 1, or 2 reports (I would
encourage you to do at least one report). They are due on or
before Tue., Feb. 7. See the assignment page and/or the Writing
Requirements handout for more
information.
We'll finish up our coverage of air pollutants today
starting with sulfur dioxide, which is considered to be the 1st air
pollutant that people became aware of. The following information
is on p. 11 in the photocopied ClassNotes.
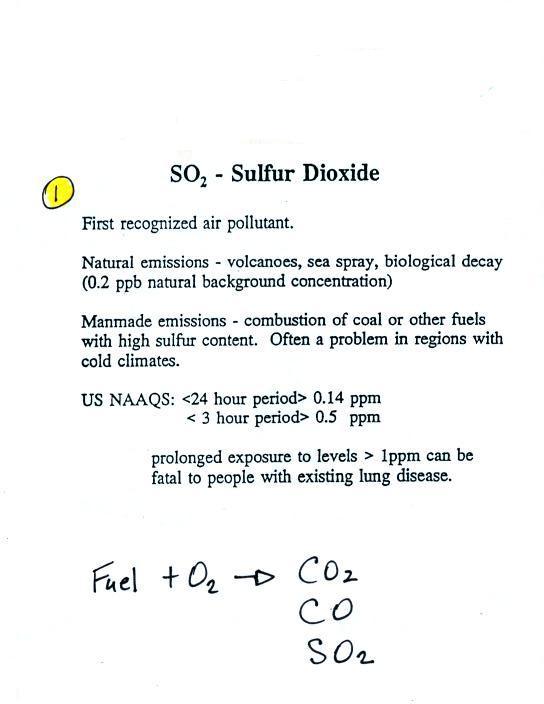
Sulfur dioxide is produced by the
combustion of sulfur-containing
fuels such as coal. The combustion also produces carbon
dioxide and carbon monoxide. People probably became aware
of sulfur dioxide because it has an unpleasant and irritating smell.
Carbon
dioxide and carbon monoxide are odorless.
Volcanoes are a natural source of sulfur dioxide.
The sulfur dioxide didn't
kill people directly. Rather it would aggravate
an existing
condition of some kind. The SO2 probably
also made people susceptible
to bacterial
infections such as pneumonia. Here's
a
link that discusses the event and its health effects in more
detail.
Some other air pollution disasters also involved high SO2
concentrations. One of the deadliest events in the US
occurred in
1948 in Donora, Pennsylvania.
"This eerie photograph was taken at noon on Oct.
29, 1948 in Donora, PA as deadly smog enveloped the town. 20 people
were asphyxiated and more than 7,000 became seriously ill during this
horrible event." The photograph below shows some of the mills
that were operating in Donora at the time. The factories were not
only emitted pollutants into the air but probably also discharging
pollutants into the river.
from: http://oceanservice.noaa.gov/education/kits/pollution/02history.html
from: http://www.eoearth.org/article/Donora,_Pennsylvania
"When Smoke Ran Like Water," a
book
about air pollution is among the books that you can check out, read,
and report on to fulfill part of the writing requirements in this class
(though I would encourage you to do an experiment instead). The
author, Devra Davis, lived in Donora Pennsylvania at the time of the
1948 air pollution episode.
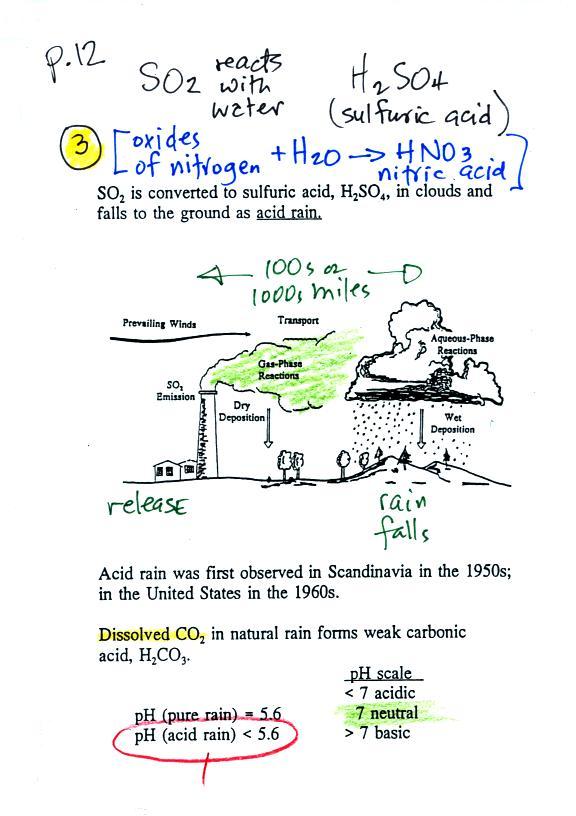
Sulfur
dioxide is one of the pollutants that can react with water in
clouds to form acid rain (some of the oxides of nitrogen can react with
water to form nitric acid). The formation and effects of acid
rain
are discussed on p. 12 in the photocopied Class Notes.
Acid rain is often a problem in regions that are
100s even 1000s of miles from the source of the sulfur dioxide that
forms the acid rain. Acid rain in Canada could come from sources
in the US, acid rain in Scandinavia came from
industrialized areas in other parts of Europe.
Note at the bottom of the figure above that natural "pristine" rain has
a pH less than 7
and is
slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. The acid rain demonstration described below and done
in class should make this point clearer.
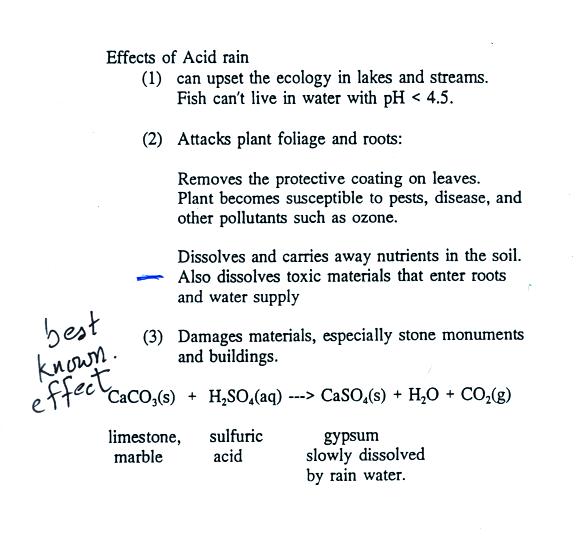
Some of the problems associated with acid rain.
Click on
this acid
rain
demonstration
link for a detailed description of the demonstration done in class.
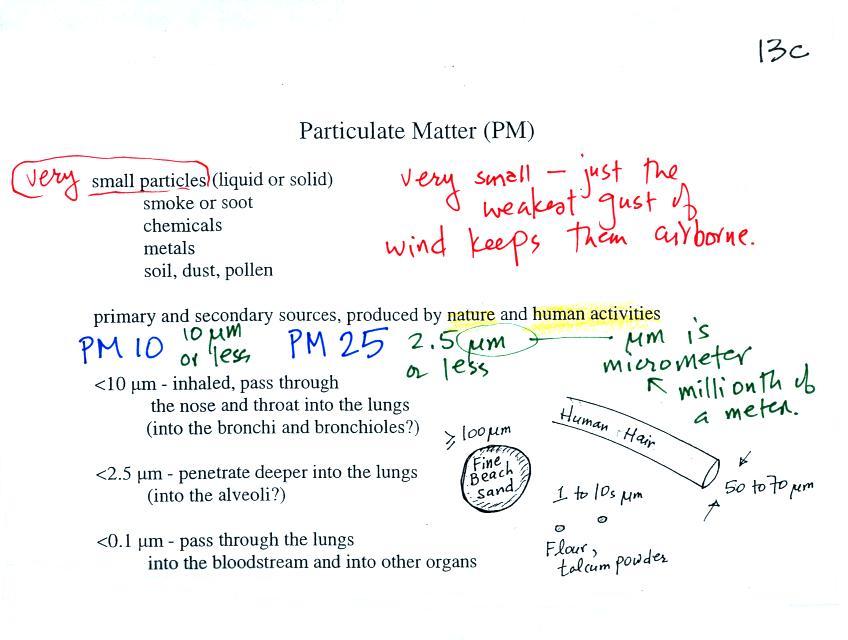
The last
pollutant that we will cover is Particulate Matter (PM) - small solid
particles or drops of liquid (but not gas) that remain suspended in
the air (particulates are sometimes referred to as aerosols). The
designations
PM10
and PM25 refer to particles with
diameters less than 10 micrometers and 2.5 micrometers,
respectively. A micrometer (µm) is one millionth of a meter
(10-6
m). The
drawing below might give you some idea of what a 1 micrometer particle
would look like (actually it would probably be too small to be seen
without magnification). You'll find some actual pictures and more
information at this source.
Red
blood
cells
are 6-10 µm
in diameter. A nanometer (nm) is 1000 times smaller
than a micrometer (10-9 m).
An atom is apparently 0.1 to 0.3
nm across, depending on the particular element.
Particulate matter can be produced
naturally (wind blown dust,
clouds above volcanic eruptions, smoke from lightning-caused forest and
brush fires). Human activities also produce particulates.
Gases sometimes react in the atmosphere to make small drops or
particles (this is what happened in the photochemical smog
demonstration). Just the smallest, weakest gust of wind is enough
to keep particles this small suspended in the atmosphere.
One of the main concerns with particulate pollution is that the small
particles might be a health hazard ( a health advisory was issued on
Sunday because of the dusty conditions in Tucson)
Particles with dimensions of 10 µm
and less can be
inhaled
into the lungs (larger particles get caught in the nasal
passages). These
inhaled particles may be poisonous, might cause cancer, damage lung
tissue, or aggravate existing
repiratory diseases. The smallest particles can pass through the
lungs and get into the blood stream (just as oxygen does) and damage
other organs in the body.
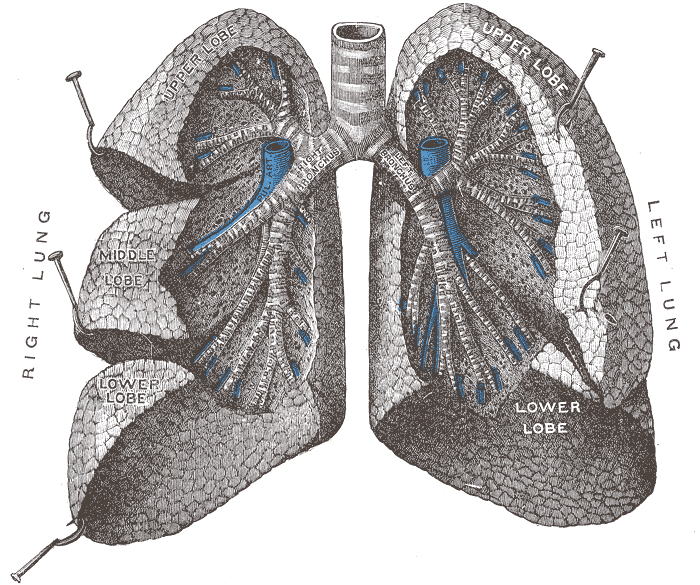
The figure below identifies some of the parts of the human lung
mentioned
in the figure above.
Crossectional view of the
human lungs
from: http://en.wikipedia.org/wiki/Lung
|
1 - trachea
2 - mainstem bronchus
3 - lobar bronchus
4 - segmental bronchi
5 - bronchiole
6 - alveolar duct
7 - alveolus
from http://en.wikipedia.org/wiki/Image:Illu_quiz_lung05.jpg
|
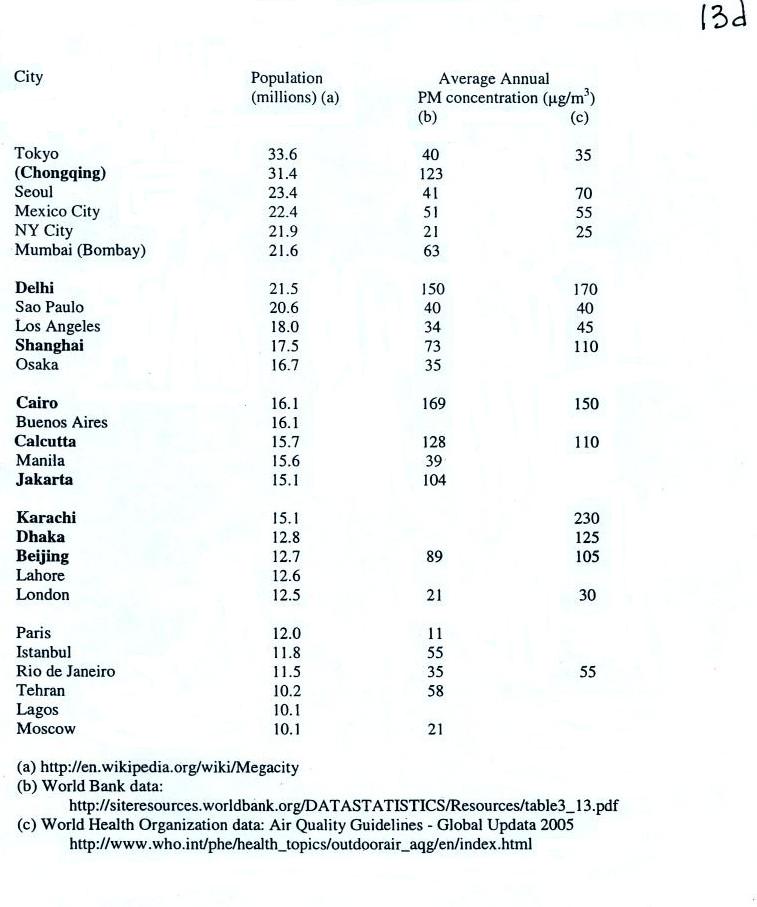
Note the
PM10 annual National
Ambient Air Quality Standard (NAAQS) value of 50 micrograms/cubic meter
at
the bottom of p. 13c in the photocopied ClassNotes (above).
The following list (p. 13d in the ClassNotes) shows that there are
several cities around the world
where PM concentrations are 2 or 3 times higher than the NAAQS
value.
There was some concern
during
the summer 2008 Olympic Games
in Beijing that the polluted air would keep athletes from performing at
their peak.
Chinese authorities restricted transportation and
industrial activities before and during the games in an attempt to
reduce pollutant concentrations. Rainy weather during the games
may have done the greatest amount of good.
Clouds and
precipitation are the
best way of cleaning pollutants from the air. We'll see later in
the semester that cloud droplets form on small particles in the air
called condensation nuclei. The cloud droplets then form
raindrops and fall to the ground carrying the particles with them.
The second main concern with particulates is the effect they may
have on visibility (esthetics should actually be spelled aesthetics -
i.e. qualities that might make something appear beautiful or not).
This could be seen last weekend.
Here's a photograph of the Catalina mountains taken just before
noon on Sunday from
the Gould Simpson Building on campus.
This
visibility Sunday morning was about as bad as I can remember.
Apparently it had been windy west of Tucson on Saturday. This
wind stirred up a lot of dust that was then carried into town.
Here's the same view taken earlier on Tuesday earlier in the week
and after some rainy weather on Monday. The air on Tuesday was
much clearner than it was on Sunday and the visibility on Tuesday was
much better.
Now we will try to understand how particulates affect
visibility (beating a concept to death again). We need to first
learn a little bit more about
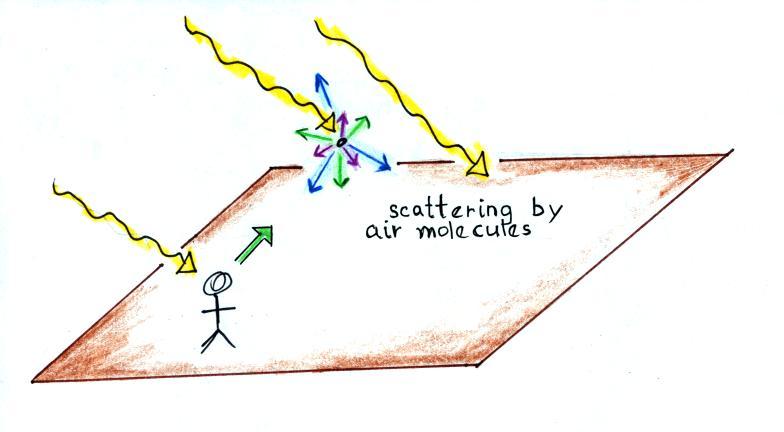
scattering. You can find all kinds of things in the sky: air,
particulates, clouds, etc. Let's first assume there isn't
anything in the sky, not even air.
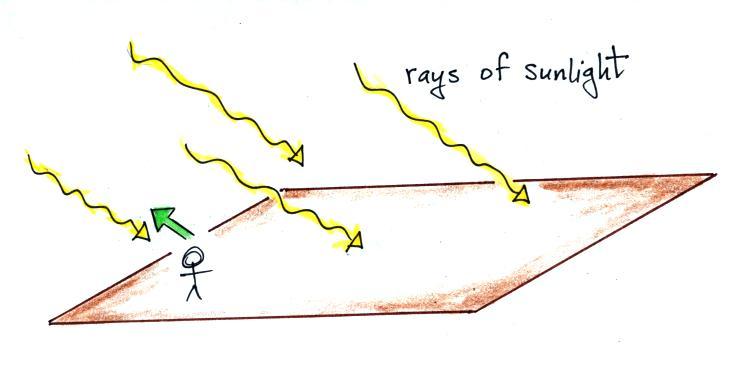
The
picture
above
shows
rays of sunlight streaming in from the
upper left. Sunlight is white light which means it is a mixture
of all the colors. I'll be using yellow to represent white light
in this and the following figures. What would you see if you
looked back along one of the rays of sunlight (the direction of the
green arrow above). You'd be looking straight at the sun and
would see the sun (of course you shouldn't do that)
What would you see if you looked away from the sun. If there
really weren't anything in the sky (no clouds, no air, no particulates,
nothing) there'd be nothing to scatter sunlight and the sky would
appear black.
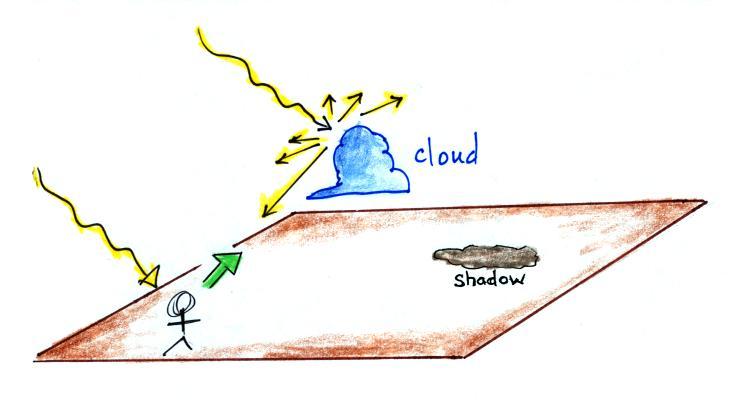
Now
we'll
put
a cloud in the sky. The small water
droplets
or
ice
crystals in a cloud scatter
and reflect sunlight. All of the colors in the beam of sunlight
are scattered equally, so the scattered light is white. That's
why clouds are (usually) white.
Air
molecules
also
scatter
light, but in a very different way.
Because they are so small
(much smaller than the wavelength of visible light) they scatter the
shorter
wavelengths (violet, blue, green) in greater amounts that the longer
wavelengths (red, orange, yellow).
Violet
has
the
shortest
wavelength and is scattered the most. However there isn't as much
violet in sunlight as there is blue and green. There's a lot of
green light in sunlight (more than any other color as a matter of fact)
but it isn't scattered as readily as blue. So the end result is
that we see blue light coming from the sky. A deep blue in this
case. The response of our eyes also plays a role. Here's a
little
more
explanation of
why the sky appears blue.
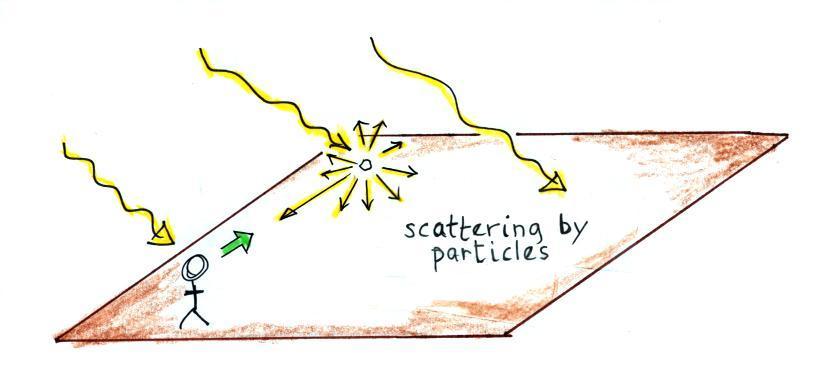
What
happens when you add particles to the air?
Particles are relatively large (compared to air molecules)
which
means
they scatter all of the colors in sunlight in equal amounts just like
cloud
droplets and ice crystals do. The
scattered light from particles is white.
So the color of the sky can change from deep blue (clean air + few
particles) to whitish blue (air molecules + more particles).
OK now let's look at how the appearance of some nearby mountains might
change as more and more particles are added to the air. We're
going to try to understand why increasing amounts of particles can
reduce visibility.
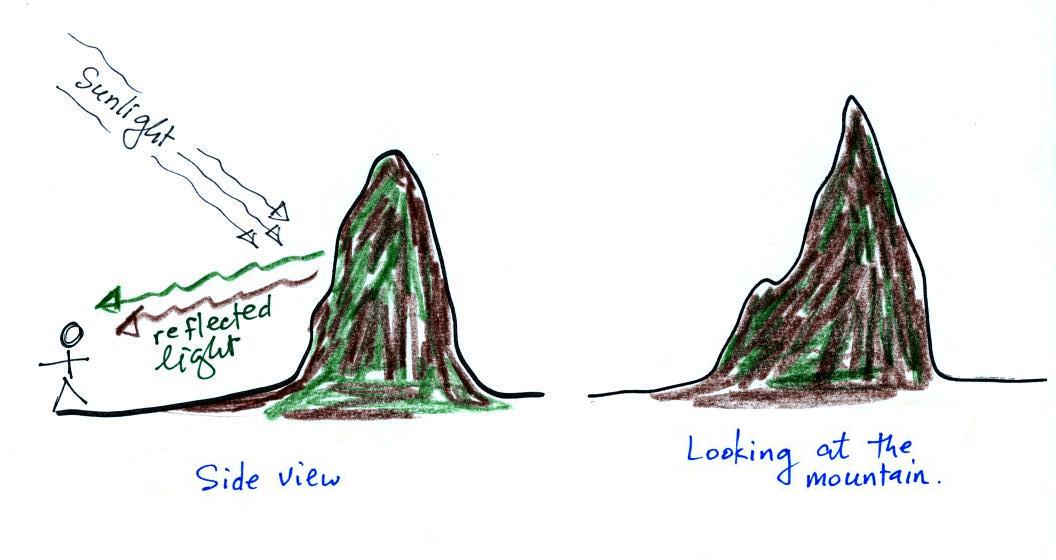
In this first picture we start out
with clean air. When we
look
at a mountain we see the light that is reflected off the soil and trees
on the mountain (shown at left above). I've colored this
reflected light green and brown. When you look at the mountain
it's green and brown (right figure above).
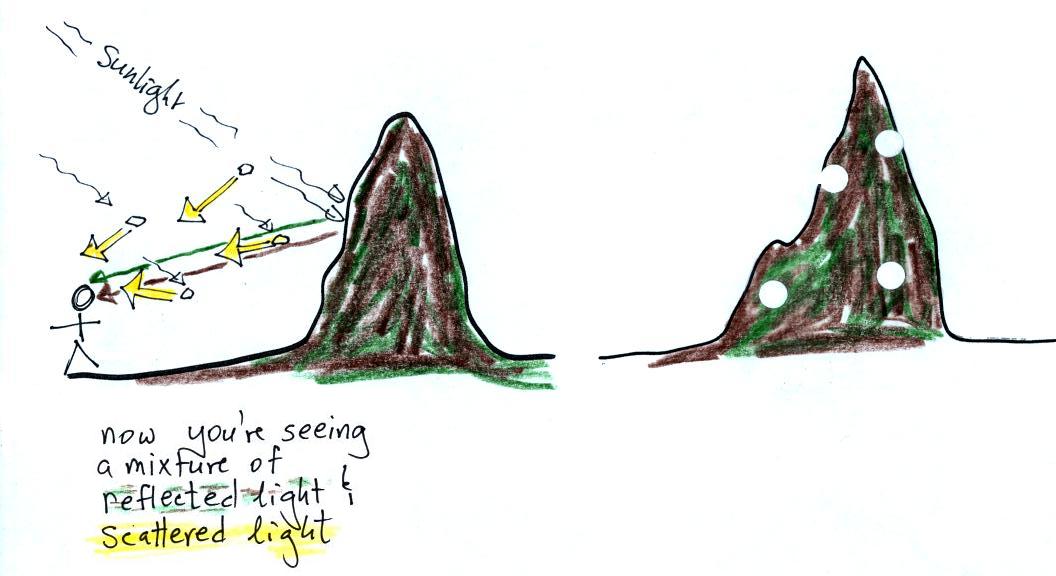
Now we'll add some particles. When you
look at the mountain
you see brown and green light plus some
white light that is coming from sunlight being scattered by the
particles (remember white light is shown in yellow in these
pictures). Some white specks of light have been superimposed on
the view of the mountain at right.
More particles, more
scattered
light, and more white light being mixed in with the brown and green
reflected light.
Even more particles. Now the
white light from scattering from particles begings to dominate.
Eventually it becomes difficult to even make out the mountain because
of all the scattered light. Light from the mountain also runs
into particles on its way toward your eyes and gets redirected so that
you don't see it. Of course there was considerable
artistic license used in this explanation (I'm also making the images a
little smaller each time for added effect).
Here are a couple of analogous situations that might help
understand how/why light scattered by particles in the air reduce
visibility.
Driving with a dirty windshield at night. Light from oncoming
traffic is scattered by dirt on the wind shield producing glare.
It is hard to see the other car and even harder to see a pedestrian or
a bicycle on the side of the road because of all the glare and
extraneous light.
Trying to understand a student in the back of the room asking a
question if lots of students in the middle and front of the room are
also talking. The students voice from the back of the room is
"drowned
out" by all the noise coming from the rest of the front (note I'm not
implying there has been a lot of noise in the classroom, quite the
opposite so far this semester)
One last
thing not
really covered in class (because I started to sense a hint
of restlessness coming from the room).
You might think that when the air is clean that visibility might
be unlimited. That isn't the case. Scattering
of sunlight by air molecules alone puts a limit on
visibility. The following figure tries to explain why this is so.
The nearby mountain appears
green
and brown.
You are
mostly seeing sunlight reflected off the mountain.
As the mountain
gets further away you start seeing increasing amounts of blue light
(sunlight scattered by air molecules in between you and the
mountain) being added to the brown and green reflected light.
This is because there is more air between you and the
mountain. The mountain at medium range now appears
brown, green, and blue. As
the mountain gets even
further away the amount of this blue light from the sky
increases.
The most distant mountain in the picture above is now blue.
Eventually the mountain gets so far away that you only see blue light
from the sky and none of the light reflected by the mountain
itself. The mountain has faded from view.
Here's a photograph of the Blue Mountains in Australia (source
of this image).
If you look closely I think you can see 5 mountain ranges in this
picture. Notice how they became fainter and fainter and lighter
and lighter blue. It is becoming hard to distinquish mountain
range 5 from the blue color of the sky.
This finishes the section on the composition of the atmosphere and
air pollutants. On Thursday we'll be moving into a completely
different topic. You can get an idea of what that will be by
navigating over to the Upcoming
Topics page.