
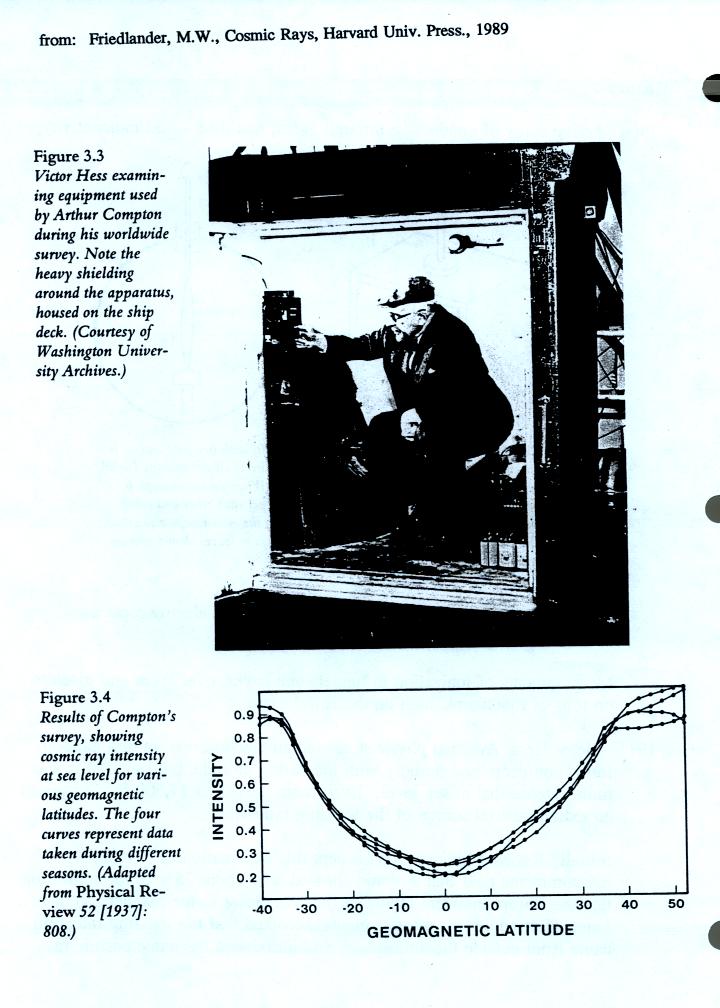
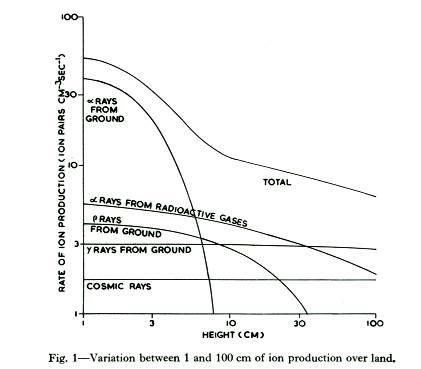
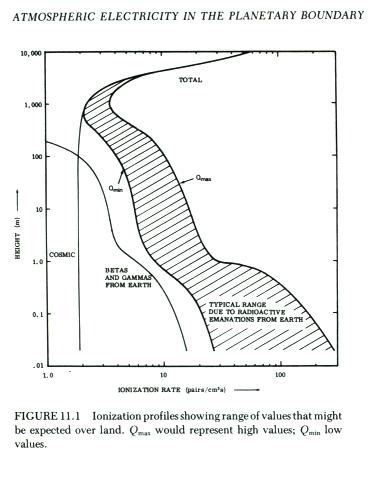
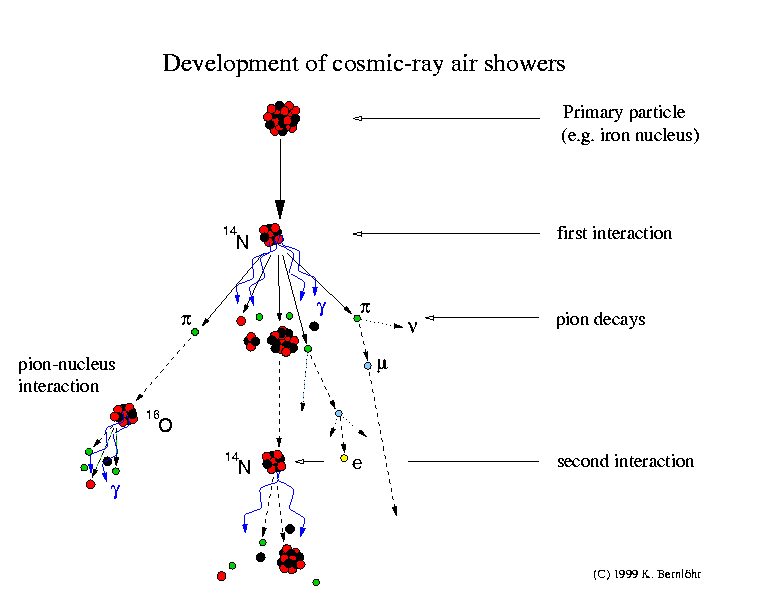
Cosmic-ray air showers
Cosmic
rays
The earth is hit by elementary particles
and atomic nuclei of very large energies. Most of them are protons
(hydrogen
nuclei) and all sorts of nuclei up to uranium (although anything
heavier than
nickel is very, very rare). Those are usually meant when talking about cosmic
rays. Other energetic particles in the cosmos are mainly electrons
and
positrons, as well as gamma-rays and neutrinos.
Interactions
and particle production
The cosmic rays will
hardly ever hit the
ground but will collide (interact) with a nucleus of the air, usually
several
ten kilometers high. In such collisions, many new particles are usually
created
and the colliding nuclei evaporate to a large extent. Most of the new
particles
are pi-mesons (pions). Neutral pions very quickly decay, usually into
two
gamma-rays. Charged pions also decay but after a longer time.
Therefore, some
of the pions may collide with yet another nucleus of the air before
decaying,
which would be into a muon and a neutrino. The fragments of the
incoming
nucleus also interact again, also producing new particles.
The gamma-rays from the
neutral pions may also create new particles, an electron and a
positron, by the
pair-creation process. Electrons and positrons in turn may produce more
gamma-rays by the bremsstrahlung mechanism.
Shower
development
The number of particles starts to
increase rapidly as this shower or cascade of particles moves downwards
in the
atmosphere. On their way and in each interaction the particles loose
energy,
however, and eventually will not be able to create new particles. After
some
point, the shower maximum, more particles are stopped than created and
the
number of shower particles declines. Only a small fraction of the
particles
usually comes down to the ground. How many actually come down depends
on the
energy and type of the incident cosmic ray and the ground altitude (sea
or
mountain level). Actual numbers are subject to large fluctuations.
In fact, from most cosmic
rays nothing comes down at all. Because the earth is hit by so many
cosmic
rays, an area of the size of a hand is still hit by about one particle
per
second. These secondary cosmic rays constitute about one third of the
natural
radioactivity.
When a primary cosmic ray
produces many secondary particles, we call this an air shower. When
many
thousand (sometimes millions or even billions) of particles arrive at
ground
level, perhaps on a mountain, this is called an extensive air shower
(EAS).
Most of these particles will arrive within some hundred meters from the
axis of
motion of the original particle, now the shower axis. But some
particles can be
found even kilometers away. Along the axis, most particles can be found
in a
kind of disk only a few meters thick and moving almost at the speed of
light.
This disk is slightly bent, with particles far from the axis coming
later. The
spread or thickness of the disk also increases with distance from the
axis.
Extensive air showers with many
particles arriving on the ground can be detected with different kinds of
particle
detectors. In the air the particles may also emit light by two
different
processes: Cherenkov light almost along the shower axis and
fluorescence light
in all directions.
Other introductory material found on
the net (HTML format):
Cosmic Rays by Richard Mewaldt
Cosmic Rays by James Schombert
Further reading found on the net (Postscript or PDF format):
Particle Data Group: review of cosmic rays
Introduction to high energy cosmic ray physics
Cosmic Ray Spectrum and Composition: Ground
Observations
This page
was written by Konrad Bernlöhr.