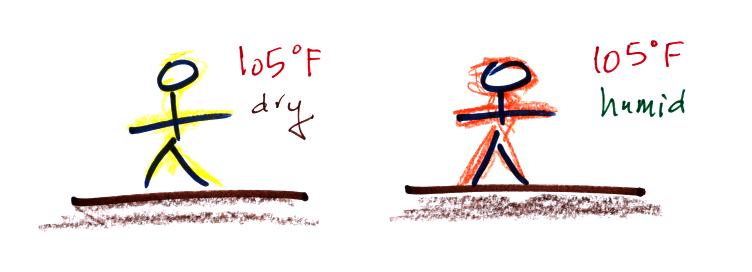Monday, Mar. 29, 2010
click here to download today's notes in
a more printer friendly format
A couple of songs before class from Calexico together with Mariachi Luz
de Luna and Francoiz Breut from an appearance at the Barbican Theater
in London before class today ("The Ballad of Cable
Hogue" and "Si
Tu Disais").
Calexico
will
be
appearing in downtown Tucson Saturday April 3
The 1S1P Bonus Assignment reports on the Causes of the Seasons were
returned today together with the In-Class Optional Assignment from last
Friday.
The Experiment #3 reports were collected today. The
Expt. #2 revised reports are due on Wednesday this week. The
Experiment #4 reports are due next Monday. You should return your
Expt. #4 materials this week and pick up the supplementary information
handout.
Here's a
quick review of a couple of things we have already covered, followed by
something "new."
A 40 F day with 30 MPH winds will feel colder (because of
increased transport of energy from your body by convection) than a 40 F
day
with no wind. The wind chill
temperature tells you how much colder it will feel.
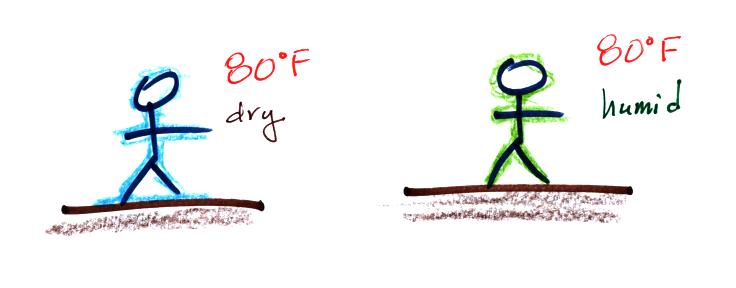
Evaporative cooling will make you feel cold if you get
out of a swimming pool on an 80 F day with dry air.
You won't feel as cold if the air is humid. Sling
psychrometers make use of this to
measure relative humidity and
dew point.
Your body tries to stay cool by perspiring. You
would still feel
hot on
a hot dry day. The heat index
measures how much hotter you'd feel on a hot humid day. The
combination of heat and high humidity is a serious weather hazard
because it can cause heatstroke
(hyperthermia).
We quickly
reviewed the progression from dry haze to wet haze to fog as the
relative humidity increases from low values to 100%. This was
stuck onto the end of the notes from last Friday.
With cold
and possibly wet weather being forecast for later in the week (preceded
by wind), you might have a chance to see
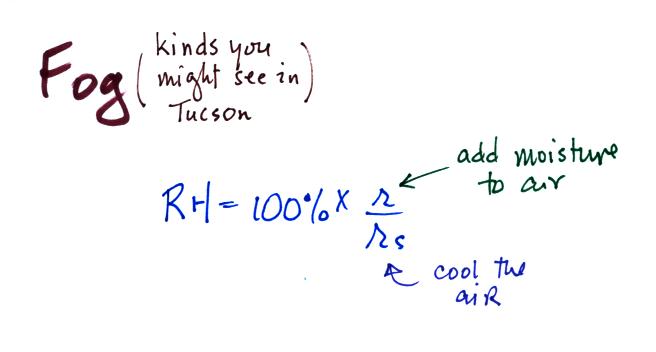
some fog in Tucson. To produce fog you first need to
increase the relative humidity (RH) to
100%
You can do this either by cooling the air (radiation fog) or
adding
moisture to
and saturating the air (evaporation or steam fog). Both will
increase the ratio in the RH formula
above.
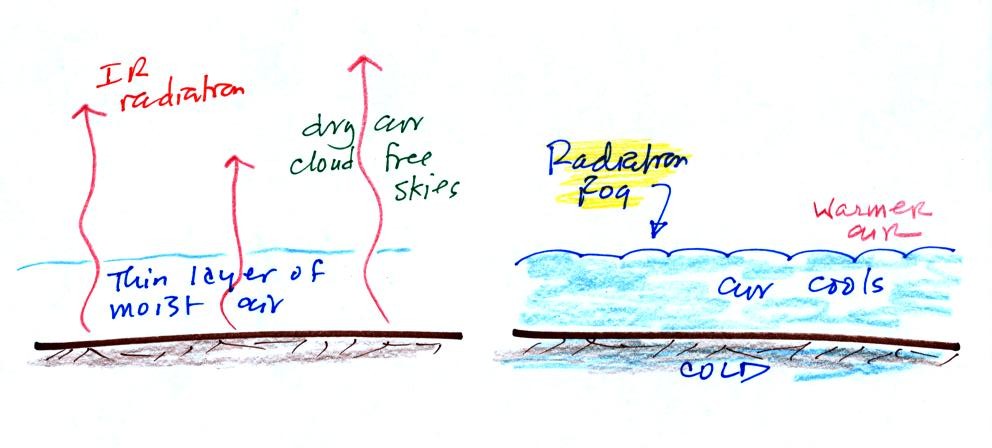
Probably the most common type of fog in Tucson is radiation fog.
The ground cools during the night by emitting IR radiation (left figure
below). The ground cools most rapidly and gets coldest when the
skies are free of
clouds and the air is dry (except for a thin layer next to the
ground.
Air in contact with the ground cools and radiation fog can form
(right
figure above). Because the fog cloud is colder than the air right
above, this is a stable situation. The fog clouds "hugs" the
ground.
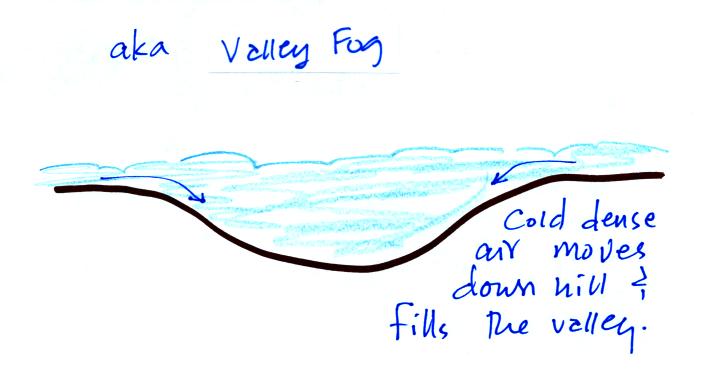
Radiation fog is sometimes called valley fog.
The cold dense foggy air will move downhill and fill low lying
areas. Because the fog reflects sunlight, it is often
difficult for the sun to warm the air
and dissipate thick clouds of valley fog.
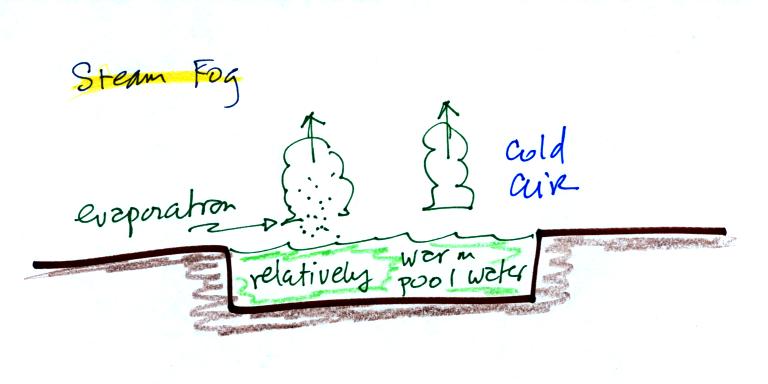
Steam fog or evaporation fog (also sometimes known as mixing fog) is
commonly observed on
cold mornings over the relatively warm water in a swimming pool.
Water evaporating from the pool
saturates the cold air above. Because the fog cloud is warmer
than the cold surrounding air, the fog clouds float upward.
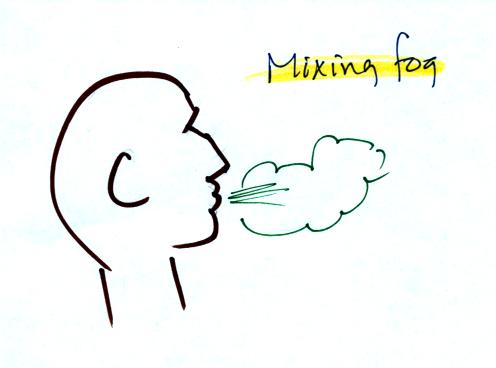
When you "see your breath" on a cold day (the figure below
wasn't shown
in class)
you're seeing mixing fog. Warm moist air from your mouth mixes
with the colder air outside. The mixture is saturated and a fog
cloud forms.
Next it was time for a demonstration that puts together many of the
concepts we have been covering. Cooling
air and
changing relative humidity, condensation nuclei, and scattering of
light are all involved in this demonstration.
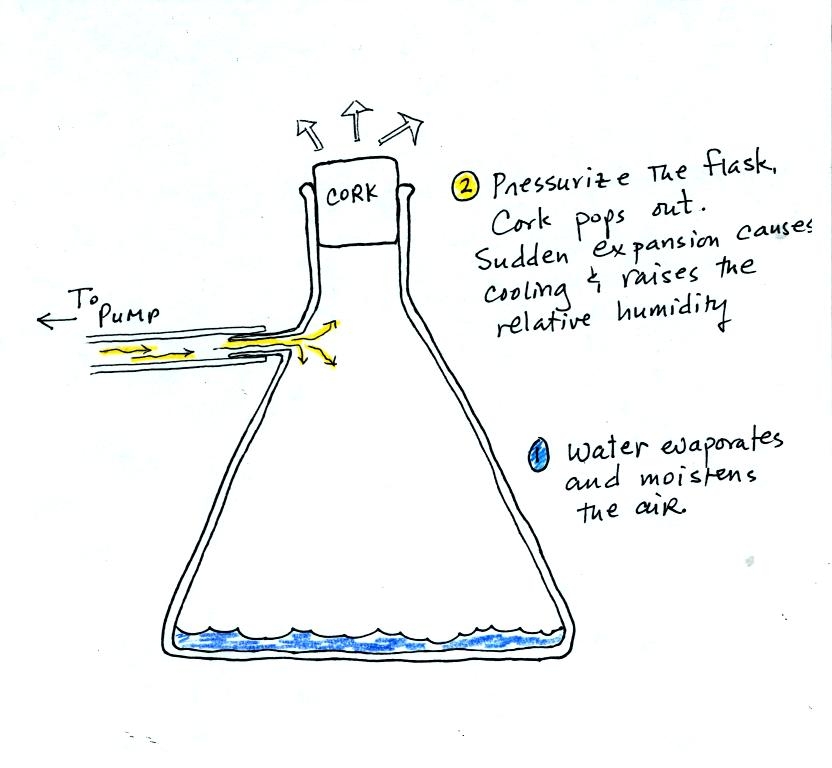
We used a strong, thick-walled, 4 liter flask (vaccum flasks
like this are designed to not implode when all of the air is pumped out
of them, they aren't designed to not explode when pressurized).
There
was a little
water in the bottom of the flask to moisten the air in the flask.
Next we pressurized the air in the flask with a bicycle pump. At
some point the
pressure blows the cork out of the top of the flask.
The air in
the flask expands outward and cools. This sudden cooling
increases the
relative humidity of the moist air in the flask to 100% ( probably more
than 100% momentarily ) and water vapor condenses onto cloud
condensation nuclei in
the air. A faint cloud became visible at this point. The
cloud droplets are too small to be seen with the human eye. You
can see the cloud because the water droplets scatter light.
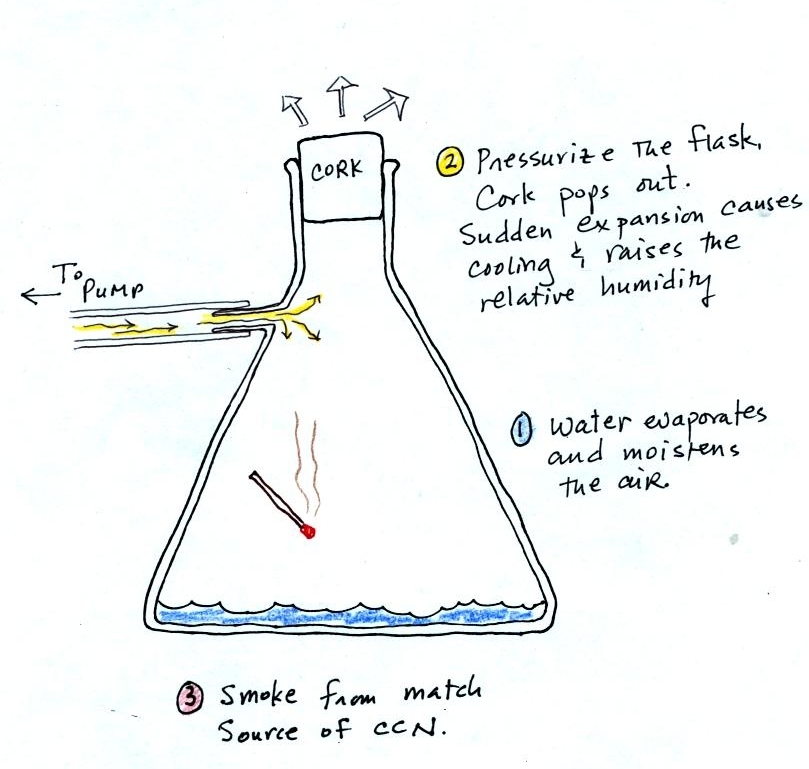
The demonstration was repeated an
additional time with one
small
change. A burning match was dropped into the
bottle. The smoke from the match added lots of very small
particles, condensation nuclei, to the air in the flask. The
cloud that formed
this time was quite a bit "thicker" and much easier to see.
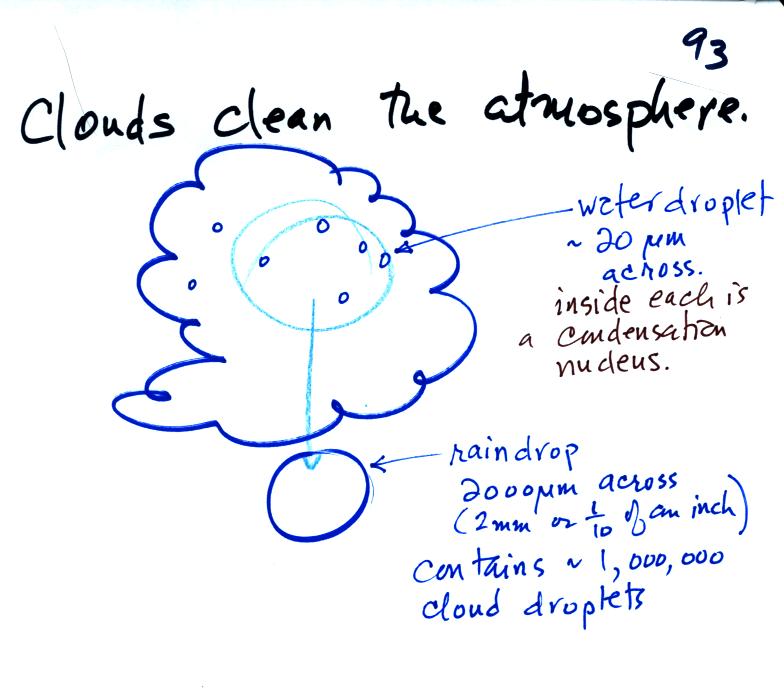
Clouds are one of the best ways of cleaning the
atmosphere
(cloud
droplets form on particles, the droplets "clump" together to form a
raindrop, and the raindrop carries the particles to the ground).
A raindrop can contain 1 million cloud droplets so a single raindrop
can remove a lot of particles from the air. You may have noticed
how clear the air seems the day after a rainstorm; distant mountains
are crystal clear and the sky has a deep blue color. Gaseous
pollutants can dissolve in the water droplets and be carried to
the ground by rainfall also.
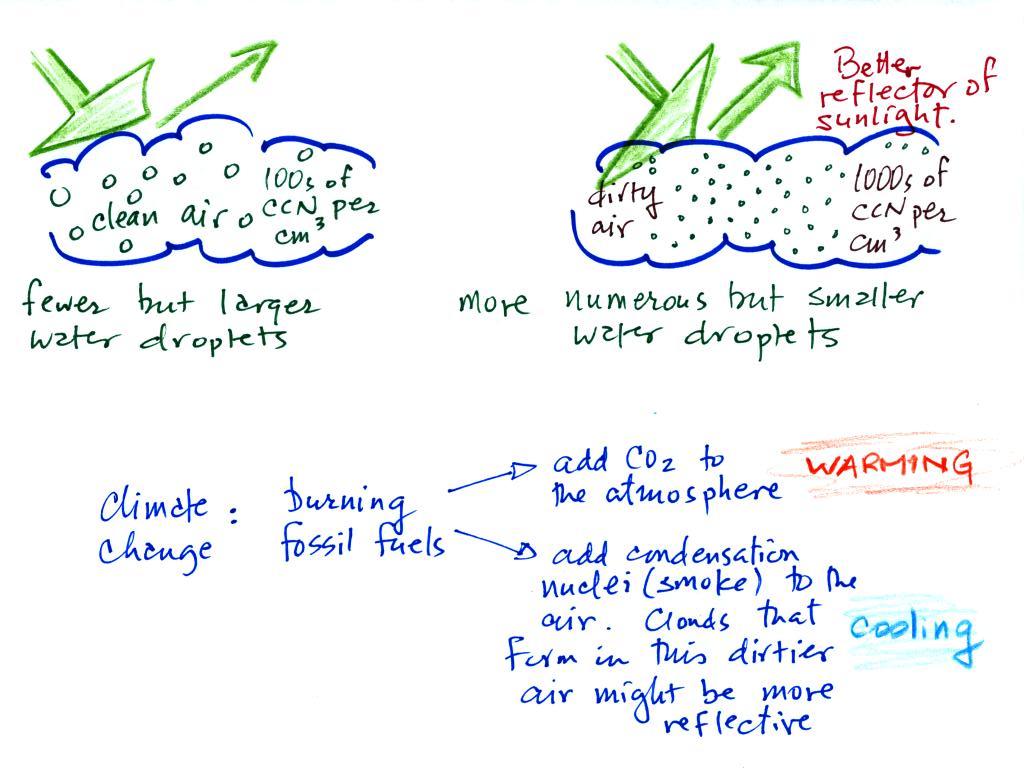
A cloud that forms in dirty air is composed of a large
number of small droplets (right figure above). This cloud is more
reflective
than a cloud that forms in clean air, that is composed of a smaller
number of larger
droplets (left figure).
Just like in the cloud-in-a-bottle demonstration, the cloud that was
created when the air was full of smoke particles was much more visible
than the cloud made with cleaner air.
This is has implications for climate change.
Combustion of fossil fuels adds carbon dioxide to the atmosphere.
There is concern that increasing carbon dioxide concentrations will
enhance the greenhouse effect and cause global warming.
Combustion also adds condensation nuclei to the atmosphere (just like
the burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form, might make
the clouds more reflective, and might cause cooling. There is
still quite a bit of uncertainty about how clouds might change and how
this
might affect climate (remember too that clouds are good absorbers of IR
radiation).
We did just get started on the next topic: cloud
identification and classification. I have moved those notes over
to the Wed., Mar. 31 online notes.