Five Facts to Memorize
The First Law of Thermodynamics (also
called the Principle of Conservation of Energy)
Energy can neither be created nor destroyed; it can however be transformed from one kind to another.
E.g., latent heat energy can be
transformed into mechanical wind energy in a convective cloud.
The Second Law of Thermodynamics (also
called the Entropy Law).
Heat will NOT flow spontaneously from a cold object to a hot object.
E.g., heat flows from the tropics
towards the poles - the basis of all weather.
A famous quote you do not need to memorize: "Thermodynamics is a funny subject. The
first time you go through it, you don't understand it at all. The second time
you go through it, you think you understand it, except for one or two points.
The third time you go through it, you know you don't understand it, but by that
time you are so used to the subject, it doesn't bother you anymore ..." -
Arnold Sommerfield.
Newton’s First Law
An object at rest will remain at rest, or will remain moving in a straight line, unless acted upon by a force(s).
E.g., A stagnant air parcel will
begin to move when a pressure gradient force is applied to it.
Newton’s Second Law
The acceleration that an object experiences is proportional to the net force applied to it, F = ma.
E.g., The strong pressure gradient force in a tornado accelerates the wind to a very high speed.
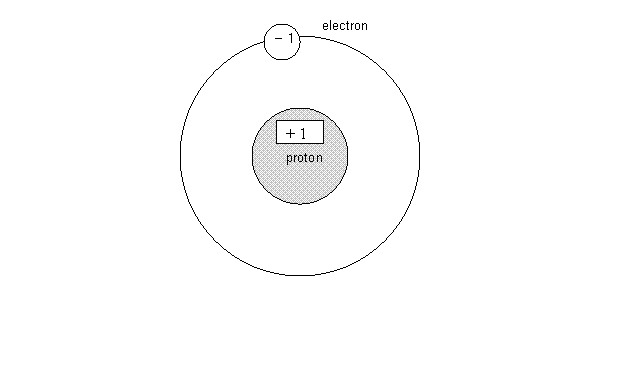
The Bohr Model of the Atom (also called the
"Planetary Model")
In the Bohr Model the positively charged
proton(s) (and neutral neutrons) occupy the central region called the nucleus,
while the negatively charged electron(s) orbit the nucleus.
E.g., when a large voltage difference is applied to a nitrogen molecule in the atmosphere the electron can be stripped off leaving two ions (a negative electron and a positive nitrogen molecule) that can conduct lightning.