Monday Feb. 25 2008
Some
material not covered in class last Friday (temperature and heat,
temperature scales) was added to the Fri., Feb.
22 online notes together with a hidden optional assignment (no
longer active). That material was discussed quickly at the
start of today's class.
Conduction is the first of four energy transport processes
that we
will cover. The figure below illustrates this process. A
hot object is stuck in the middle of some air.
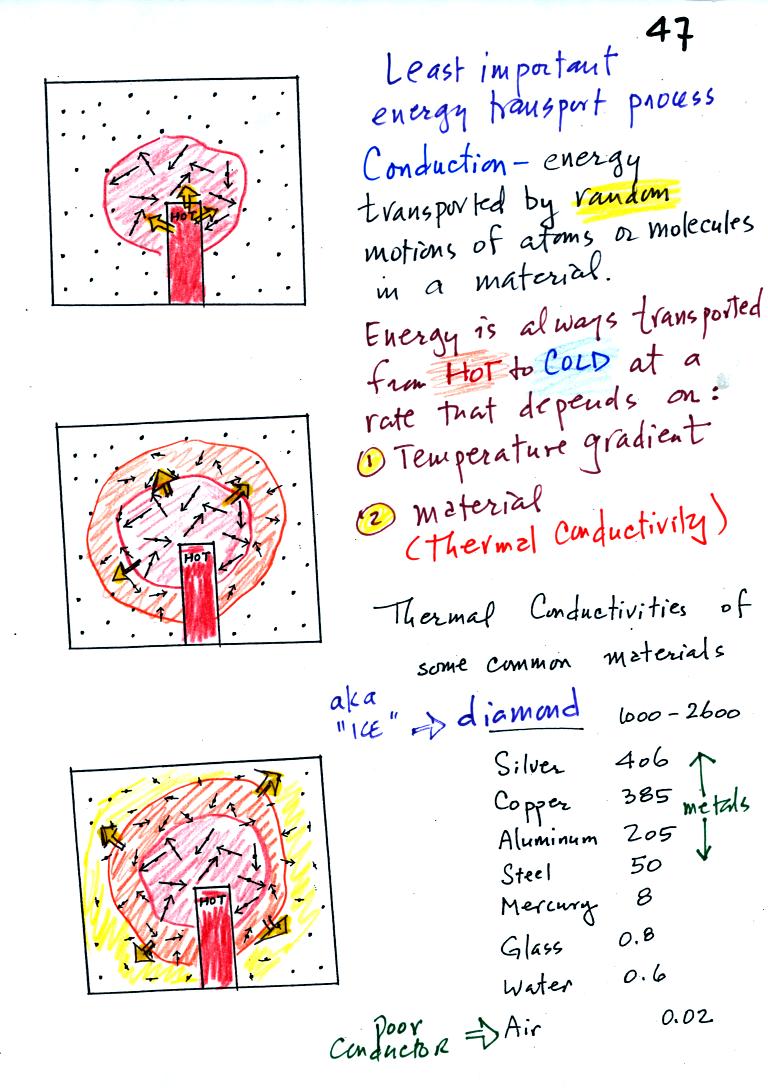
In the top picture some of the atoms or molecules near the
hot object have collided with the object and picked up energy from the
object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red).
In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object.
In
the third picture molecules further out have now (the yellow ones)
gained
some energy. The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
Conduction transports energy from hot to cold. The rate of
energy transport depends first on the material (air in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (sauce pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
The following three figures show a demonstration
that was performed in class. Curry powder was used instead of
acetic acid (concentrated acetic acid is too dangerous; contact with
the vapor causes eye burns and irreversible eye damage, severe
irritation of the respiratory tract, and corrosion of the digestive
tract).
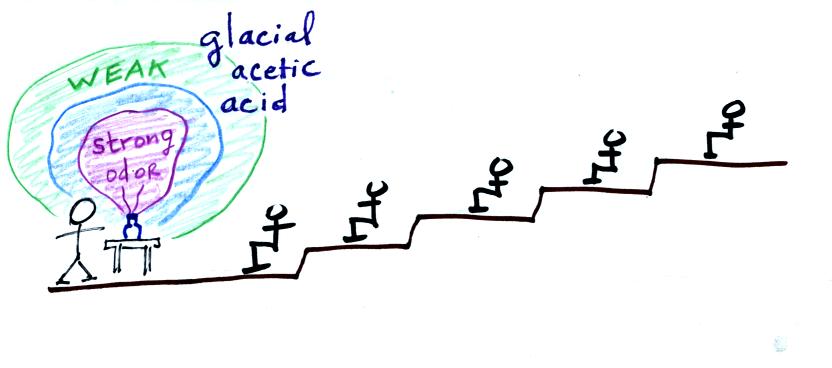
As the smell of the curry gets into the air,
collisions with air molecules begin to move the smell toward the back
of the room.
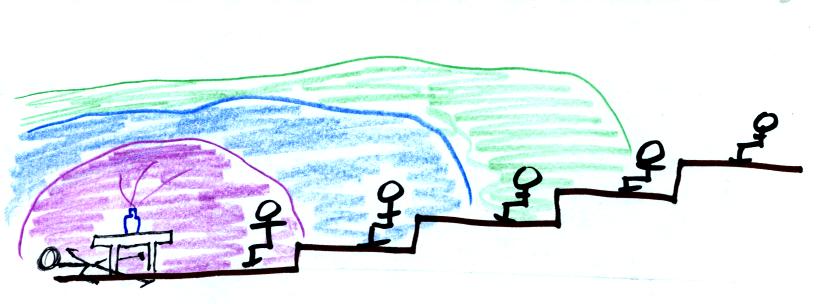
If acetic acid had been used the instructor (and perhaps the front row
of students) might have lost consciousness by this point.
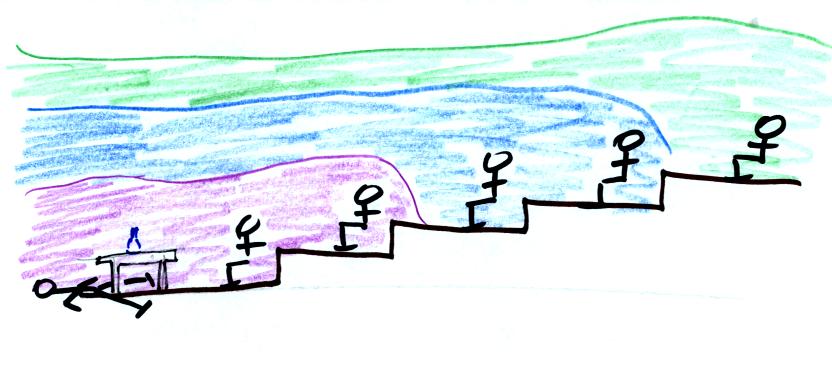
later still
Because
air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection (we'll look at convection
shortly). Here are some examples of
insulators that use air:
Small bubbles of air trapped in foam
Thin insulating layer of air
Hollow fibers (Hollofil) filled with air used in sleeping bags and
winter coats
Convection
was the next energy transport process we had a look at. Rather
than moving about randomly, the atoms or molecules move as a
group. Convection works in liquids and gases but not solids.
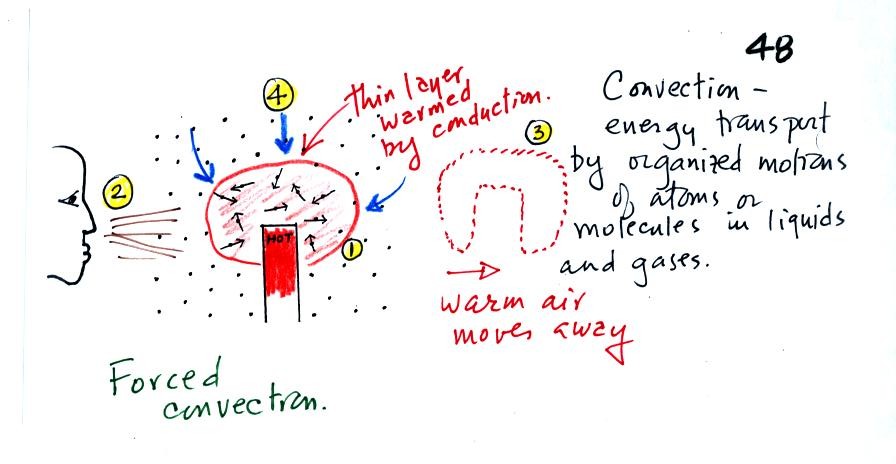
At Point 1 in the picture above a thin layer of air
surrounding a hot object has
been
heated by conduction. Then at Point 2 a person (yes that is a drawing
of a
person's head) is blowing the blob of warm air
off to the right. The warm air molecules are moving away at Point
3 from the
hot object together as a group (that's the organized part of the
motion). At Point 4 cooler air moves in and surrounds the hot
object and the cycle can repeat itself.
This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly.
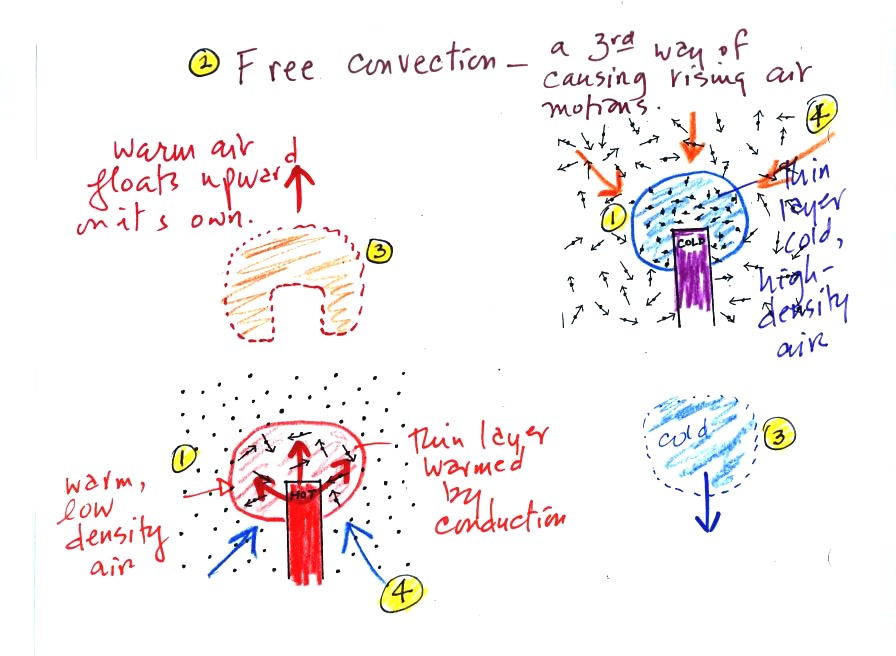
A thin layer of air at Point 1 in the figure above (lower
left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands as it
moves into lower pressure surroundings and cools. If the air is
moist, clouds can form). Cooler air moves in to take the place of
the rising air at Point 4 and the process repeats itself.
The example at upper right is also free convection. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.
Now some
practical applications of what we have learned about conductive and
convective energy transport. Energy transport really does show up
in a lot more real life situations than you might expect.
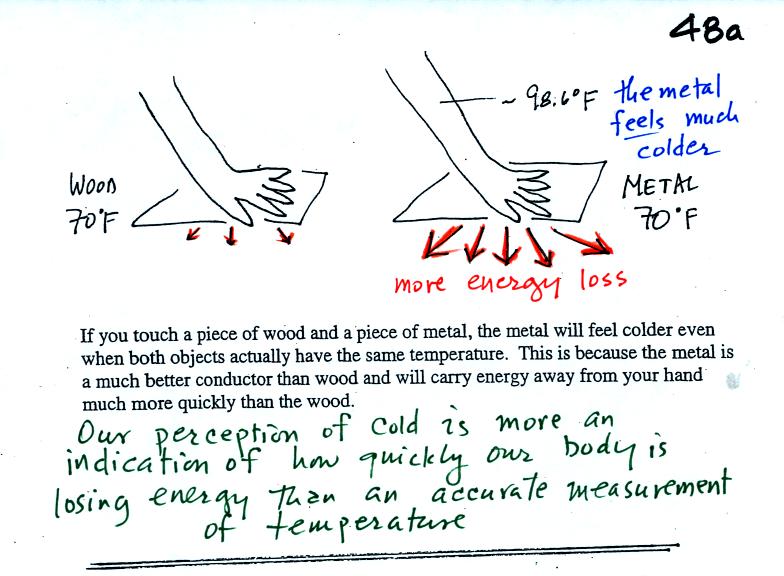
Note first of all there is a temperature difference between
your hand and a 70o F object. Energy will flow from your warm
hand to the colder object. Metals are better conductors than
wood. If you touch a
piece of
70 F metal it will feel much colder than a piece of 70 F wood. A
piece
of 70 F diamond would feel even colder because it is a better conductor
than metal. Something that feels cold may not be as
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
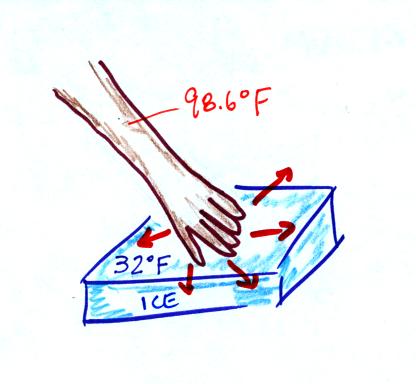
The following picture wasn't shown
in class. Ice fells cold even though is not a particularly
good conductor. In this case a lot of energy is flowing from your
hand to the ice because there is a much larger temperature
difference. This high rate of energy loss causes ice to feel cold.
Air is a poor conductor. If you stick your hand out in
40 F
weather the air won't conduct energy away from your hand very quickly
at all and the air won't feel very cold.
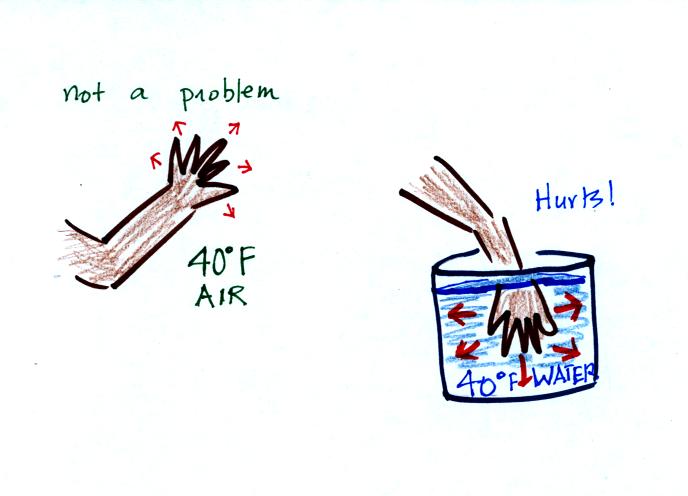
If you stick your hand
into a bucket of 40 F water, it will feel very cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water.
Now we're
in a perfect position to understand wind chill.
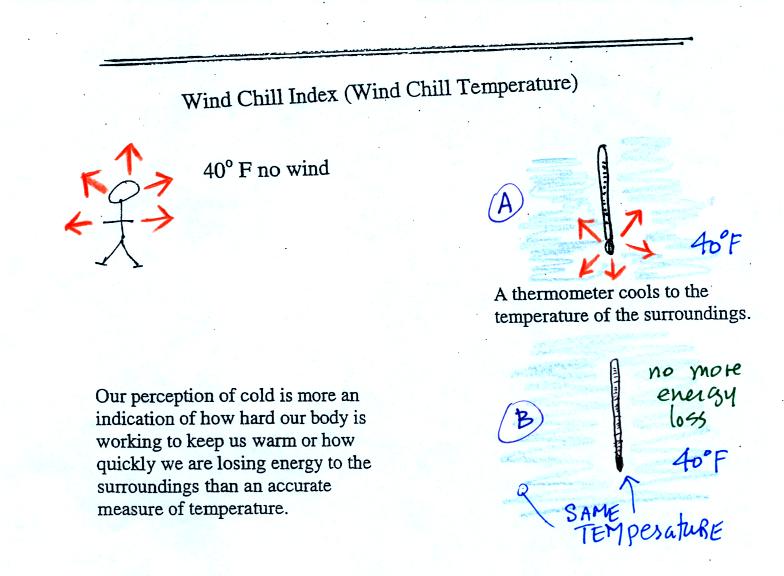
If you go outside on a 40 F day (calm winds) you will feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). A thermometer
behaves differently. It actually cools to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy.
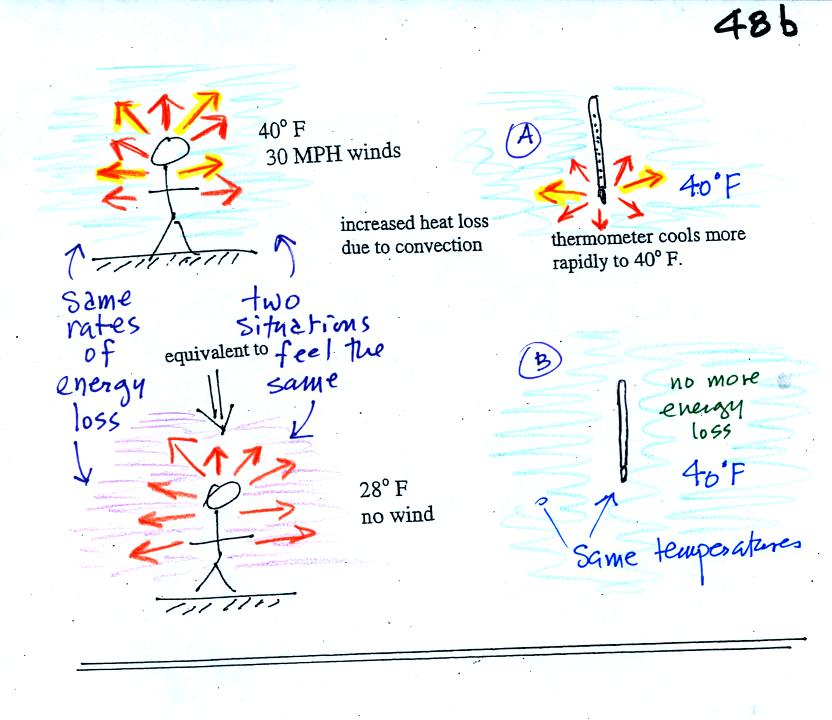
If you go outside on a 40 F day with 30 MPH winds your body
will lose
energy at a more rapid rate (because convection together with
conduction are transporting energy away from your body). This
higher rate of energy loss will make it feel colder than a 40
F day
with calm winds.
Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a calm 28
F day. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
Standing outside on a 40 F day is usually not a life threatening
situation. Falling into 40 F water is.
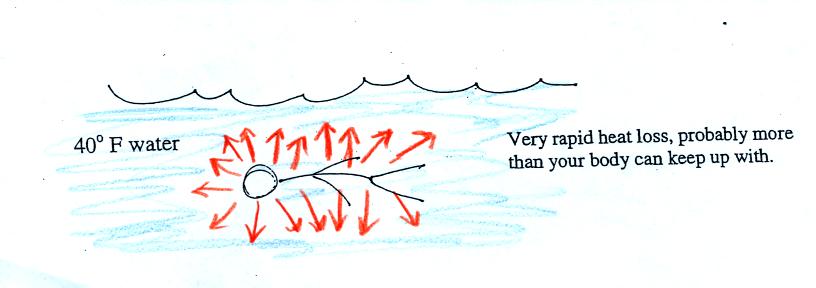
Energy will be conducted away from your body more quickly than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia which
can bring on
heatstroke and which is also a serious outdoors risk in S.
Arizona. Don't confuse either of the links above with the link to
a hidden optional assignment.














