Wednesday, Jan. 23, 2019
Sia: "California
Dreamin' " (3:37), "Chandelier"
(4:14), "Chandelier"
(4:05), "Midnight
Decisions" (3:44), "Breathe
Me" (4:55), "The
Girl You Lost to Cocaine" (3:58)
We'll be using page 23a,
page 23b, page 23c, page 23d, page 24a, and perhaps page 29 today from the
ClassNotes.
An Optional
(Extra Credit) Assignment was handed out in class today and
collected at the end of class. If you weren't in class, you
can download the assignment and turn it in at the beginning of
class on Friday.
Mass,
weight, density, and pressure.
Pressure
is a pretty important concept, that's what
we'll be working on today. Differences
in atmospheric pressure create winds which can
then cause storms. To better understand
pressure we need to first review mass and
weight.
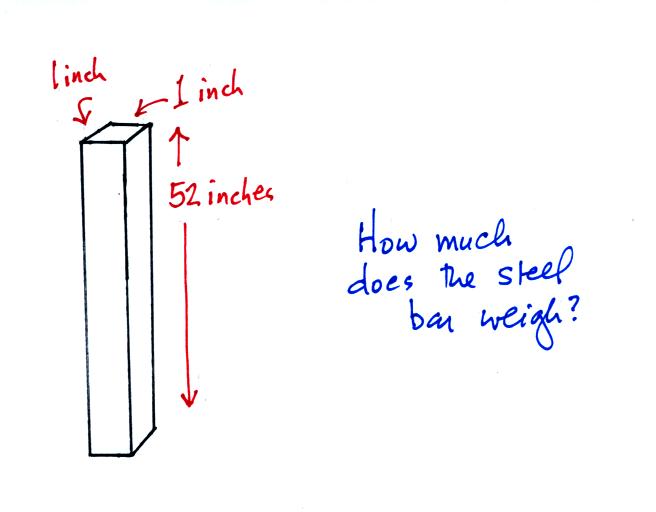
Weight is something you can feel. I'll
pass an iron bar around in class (it's
sketched below) - lift it and try to guess
it's weight. The fact that it is a 1" by
1" is significant. More about the bar
later in today's notes.
I used to
pass around a couple of small plastic bottles (see
below). One contained some water, the other an equal
volume of mercury (here's the source
of the nice photo of liquid mercury below at
right). I wanted you to appreciate how much
heavier and denser mercury is compared to
water.
But the plastic bottles have a way of getting brittle with
time and if the mercury were to spill in the classroom the
hazardous material people would need to come in and clean it
up. That would probably take a few days, would be very
expensive, and I would get into a lot of trouble. So
this semester I'll pass around a smaller, much safer, sample
of mercury so that you can at least see what mercury it looks
like (it's a recent purchase from a company in London).
I'll keep the plastic bottles of mercury up at the front of
the room just in case you want to see how heavy the stuff is.
It
isn't so much the liquid mercury that is a hazard, but
rather the mercury vapor. Mercury vapor is used in
fluorescent bulbs (including the new energy efficient CFL
bulbs) which is why they need to be disposed of properly
(you shouldn't just throw them in the dumpster).
That is a topic that will come up again later in the
class. Mercury
and bromine are the only two elements that are found
naturally in liquid form. All the other elements are
either gases or solids.
I am hoping that you will remember and understand the
following statement
atmospheric
pressure at any level in the atmosphere
depends on (is determined by)
the weight
of the air overhead
We'll
first review the concepts of mass, weight, and density
but understanding pressure is our main goal.
I've numbered the various sections to help with
organization. There's a summary at the end of
today's notes.
1.
weight
This is a good place to start because this is
something we are pretty familiar with. We
can feel weight and we routinely measure weight.
A person's weight also depends
on something else.
In outer space away from
the pull of the earth's gravity people are weightless.
Weight depends on the person and on the pull of gravity.
We
measure weight all the
time. What units
do we use?
Usually pounds, but
sometimes ounces or
maybe tons.
Students sometimes
mention Newtons, those
are metric units of
weight (force).
2. mass
Rather than just saying the
amount of something it is probably better to use the
word mass
It would be possible to have equal volumes of
different materials or the same total number of atoms or
molecules of two different materials, and still have different
masses.
Grams (g) and kilograms (kg) are commonly used units of
mass (1 kg is 1000 g). They're metric units (slugs are
the units of mass in the English (American) system of units).
3. gravitational
acceleration

On the surface of the earth, weight is
mass times a constant, g, known as the
gravitational acceleration. The value of g
is what tells us about the strength of gravity on the earth;
it is determined by the size and mass of the earth. On
another planet the value of g would be
different. If you click here
you'll find a little (actually a lot) more information about
Newton's Law of Universal Gravitation. You'll see how
the value of g is determined and why it is called
the gravitational acceleration. These aren't details
you need to worry about but they're there just in case
you're curious.
Here's a question to test your understanding.

The masses are all the same. On the earth's surface the
masses would all be multiplied by the same value of g.
The weights would all be equal. If
all 3 objects had a mass of 1 kg, they'd all have a weight of 2.2
pounds. That's why we can use
kilograms and pounds interchangeably.
The following figure show a situation where two
objects with the same mass would have different weights.

On the earth a brick has a mass of about
2.3 kg and weighs 5 pounds. If you were to travel to the
moon the mass of the brick wouldn't change (it's the same
brick, the same amount of stuff). Gravity on the moon is
weaker (about 6 times weaker) than on the earth because the
moon is smaller and it has less mass, the value of g
on the moon is different than on the earth. The brick
would only weigh 0.8 pounds on the moon.
The brick would weigh almost 12 pounds on the surface on
Jupiter where gravity is stronger than on the earth.
On the moon, a brick would have the same mass, the same
volume, the same density, but a different weight as(than)
it would on the earth.
The three objects below
were not passed around class (one of them is pretty
heavy). The three objects all have about
the same volumes. One is a piece of wood,
another a brick, and the third is something
else.
The
easiest way to determine which is which is to lift each
one. One of them weighed about 1 pound (wood), the 2nd
about 5 pounds (a brick) and the last one was 15 pounds (a
block of lead).
The point of all this was to get you thinking about
density. Here we had three objects of about
the same size with very different weights. Different
weights means the objects have different masses (since weight
depends on mass). The three different masses, were
squeezed into roughly the same volume producing objects of
very different densities.
4. density
The brick is in the back, the lead
on the left, and the piece of wood (redwood) on the right.
The wood is less dense than water (see the table below) and
will float when thrown in water. The brick and the lead
are denser than water and would sink in water.
We'll be more concerned with air in this
class than wood, brick, or lead.
In the first example
below we have two equal volumes of air but the amount
(mass) of air squeezed into each volume is different (the
dots represent air molecules).
The amounts of air (the masses) in the second example are the
same but the volumes are different. The left example
with air squeezed into a smaller volume has the higher
density.
material
|
density g/cc
|
air
|
0.001
|
redwood
|
0.45
|
water
|
1.0
|
iron
|
7.9
|
lead
|
11.3
|
mercury
|
13.6
|
gold
|
19.3
|
platinum
|
21.4
|
iridium
|
22.4
|
osmium
|
22.6
|
g/cc = grams per cubic centimeter
cubic centimeters are units of volume - one cubic
centimeter is about the size of a sugar cube
1 cubic centimeter is also 1 milliliter (mL)
I would sure like to get my hands on a brick-size
piece of iridium or osmium just to be able to feel how
heavy it would be - it's about 2 times denser than
lead.
Here's a more subtle concept. What if you were in outer
space with the three wrapped blocks of lead, wood, and
brick? They'd be weightless.
Could you tell them apart then? They would still have very
different densities and masses but we wouldn't be able to feel how
heavy they were.
5.
inertia
I think the following illustration will
help you to understand inertia.
Two stopped cars. They are the same size except
one is made of wood and the other of lead. Which
would be hardest to get moving (a stopped car resists
being put into motion). It would take considerable
force to get the lead car going. Once the cars are
moving they resist a change in that motion. The
lead car would be much harder to slow down and stop.
This is the way you could try to distinguish
between blocks of lead, wood, and brick in outer space.
Give them each a push. The wood would begin moving more
rapidly than the block of lead even if both are given
the same strength push.
I usually
don't mention in class that this concept of
inertia comes from Newton's 2nd law of motion
F = m a
force = mass x acceleration
We can rewrite the equation
a = F/m
This shows cause and effect more clearly. If you exert a
force (cause) on an object it will accelerate (effect).
Acceleration can be a change in speed or a change in direction (or
both). Because the mass is in the denominator, the
acceleration will be less when mass (inertia) is large.
Here's where we're at
From left to right the brick, the iron bar, the piece
of wood, and the lead block. They're all standing on end.
The weight of the iron bar is still unknown.
Now
we're close to
being ready to
define (and
hopefully
understand)
pressure.
It's a pretty
important
concept.
A lot of what
happens in the
atmosphere is
caused by
pressure
differences.
Pressure
differences
cause
wind.
Large pressure
differences
(such as you
might find in
a tornado or a
hurricane) can
create strong
and
destructive
storms.
The air that surrounds the earth has mass. Gravity pulls
downward on the atmosphere giving it weight. Galileo
conducted a
simple experiment to prove that air has weight (in the
1600s).
We
could add a very
tall 1 inch x 1
inch column of air
to the
picture.
Other than being a
gas, being
invisible, and
having much lower
density, it's
really no
different from the
other objects.
6. pressure
Atmospheric pressure at
any level in the atmosphere
depends on (is determined
by)
the weight of the air
overhead
This
is one way, a sort of large scale, atmosphere size
scale, way of understanding air pressure.
Pressure depends on, is determined by, the weight of the
air overhead. To determine the pressure you need to
divide by the area the weight is resting on.
and here we'll apply the
definition to a column of air stretching from sea
level to the top of the atmosphere (the figure below
is on page 23d
in the ClassNotes)
Pressure is defined as force divided by area. Atmospheric
pressure is the weight of the air column divided by the area at
the bottom of the column (as illustrated above).
Under normal conditions a 1 inch by 1 inch column of air
stretching from sea level to the top of the atmosphere will weigh
14.7 pounds.
Normal atmospheric pressure at sea level is 14.7 pounds per square
inch (psi, the units you use when you fill up your car
or bike tires with air).
Now back to the iron bar. The bar actually weighs
14.7 pounds (many people I suspect think it's heavier than
that). When you stand the bar on end, the pressure at
the bottom would be 14.7 psi.
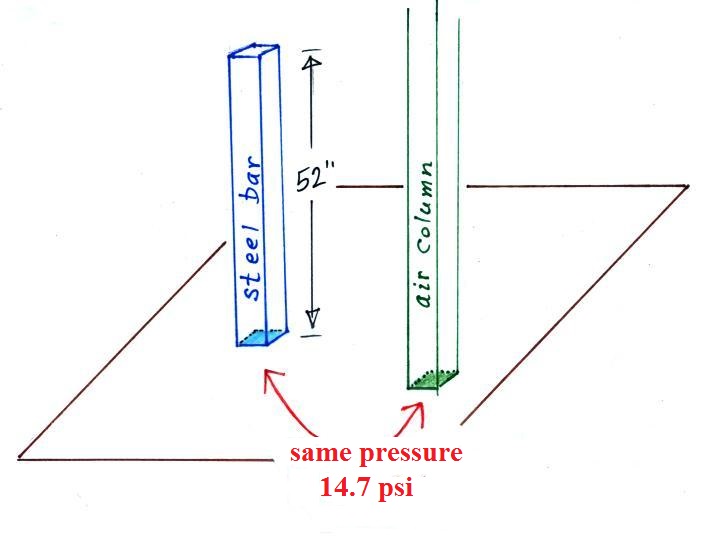
The weight of the 52 inch
long 1" x 1" steel bar is the same as a 1" x 1" column
of air that extends from sea level to the top of the
atmosphere 100 or 200 miles (or more) high. The
pressure at the bottom of both would be 14.7 psi.
7. pressure units
Pounds per square inch, psi, are
perfectly good pressure units, but they aren't the ones
that meteorologists use most of the time.
Typical sea
level pressure is 14.7 psi or about 1000 millibars
(the units used by meteorologists and the units that we will
probably mostly use in this class) or about 30 inches of
mercury (refers to the reading on a mercury
barometer). Milli means 1/1000 th. So
1000 millibars is the same as 1 bar. You sometimes see
typical sea level pressure written as 1 atmosphere.
Summary

Average and record
setting sea level pressure values
Sea level pressure averages about 1000 mb but it changes
and can be higher or lower than that.
The simple figure above at left contains
just the essential information. Focus on and try to
remember that. A lot more information and details have
been added to the figure at right.
Sea level pressure values usually fall between 950 mb and
1050 mb.
Record HIGH level sea pressure values occur during cold
winter weather. The TV weather forecast
will often associate hot weather with high pressure.
This might seem contradictory but they are generally
referring to upper level high pressure (high pressure at
some level above the ground) rather than surface pressure.
You'll sometimes hear this upper level high pressure
referred to as a ridge, we'll learn more about this later in
the semester.
Record setting LOW sea level pressure values are found in
the centers of strong hurricanes.
Hurricane Wilma in 2005 set a new record low sea level
pressure reading for the Atlantic, 882 mb. Hurricane
Katrina earlier in the same year had a pressure of 902
mb. The table below lists some information on intense
hurricanes. 2005 was a very unusual year, 3 of the 10
strongest N. Atlantic hurricanes ever occurred in
2005. There were also a record number of Atlantic
hurricanes in 2005. The strongest Atlantic
hurricanes from 2017 have been added. 2017 was the
costliest hurricane season on record in the United States
and the deadliest since 2005.
Hurricane Patricia off the west coast of Mexico in fall
2015 set a new surface low pressure record for the Western
Hemisphere - 872 mb and very close to the world
record. Sustained winds of 200 MPH were observed in
that storm.
Most
Intense North Atlantic Hurricanes
|
Most
Intense Hurricanes
to hit the US Mainland
|
Wilma
(2005) 882 mb
Gilbert (1988) 888 mb
1935 Labor Day 892 mb
Rita (2005) 895 mb
Allen (1980) 899
Katrina (2005) 902
|
1935
Labor Day 892 mb
Camille (1969) 909 mb
Katrina (2005) 920 mb
Andrew (1992) 922 mb
1886 Indianola (Tx) 925 mb |
strong 2017 hurricanes
|
Harvey 937 mb
Irma 914 mb
Jose 938 mb
Maria 908 mb
|
What makes hurricane winds so strong is the pressure gradient,
i.e. how quickly pressure changes with distance (horizontal
distance). Pressure can drop from near average values
(1000 mb) at the edges of the storm to the low values shown
above at the center of the storm.
Low pressure is also found in the centers of
tornadoes. A surface pressure value of 850 mb was
measured in 2003 inside a strong tornado in Manchester, South
Dakota (https://www.weather.gov/fsd/20030624-tornadosamaras).
This is a very difficult (and very dangerous) thing to try to
do. Not only must the instruments be built to survive a
tornado but they must also be placed on the ground ahead of an
approaching tornado and the tornado must then pass over the
instruments (also the person placing the instrument needs to
get out of the way of the approaching tornado).
You can experience pressure values much lower than that
(roughly 700 mb) by simply driving up to Mt. Lemmon.
Pressure changes much more quickly if you move vertically in
the atmosphere than if you move sideways. Very strong
vertical changes in pressure are usually almost balanced
exactly by gravity.