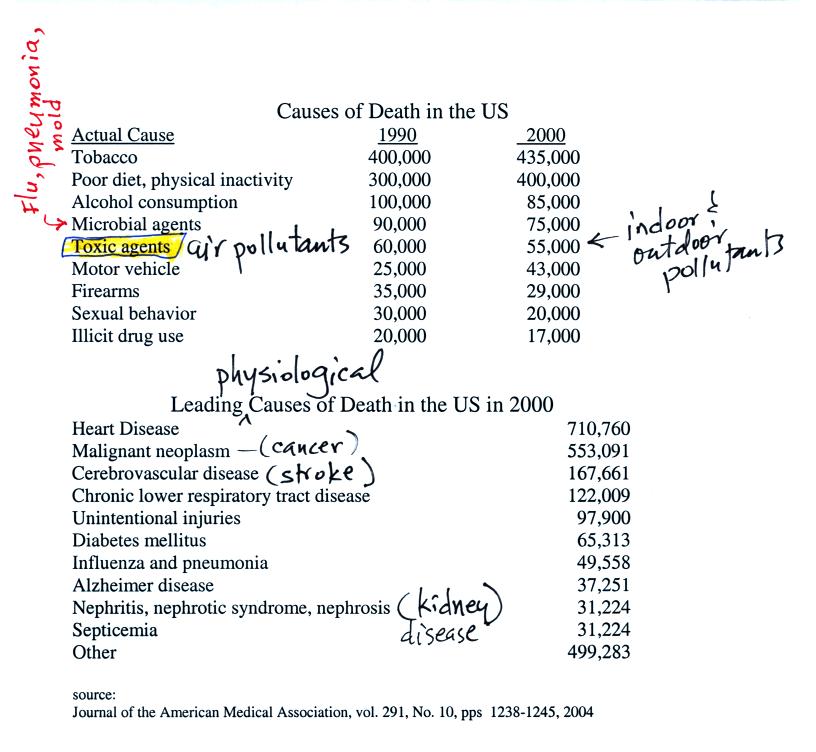
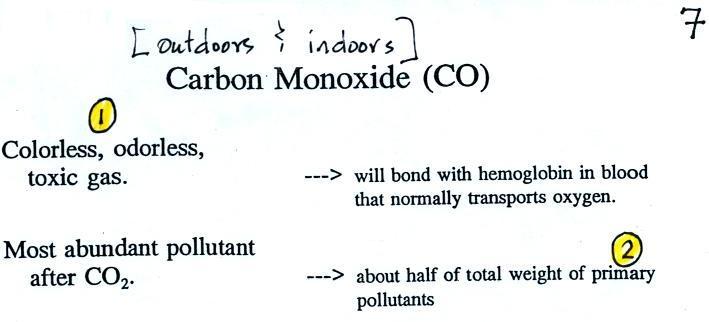
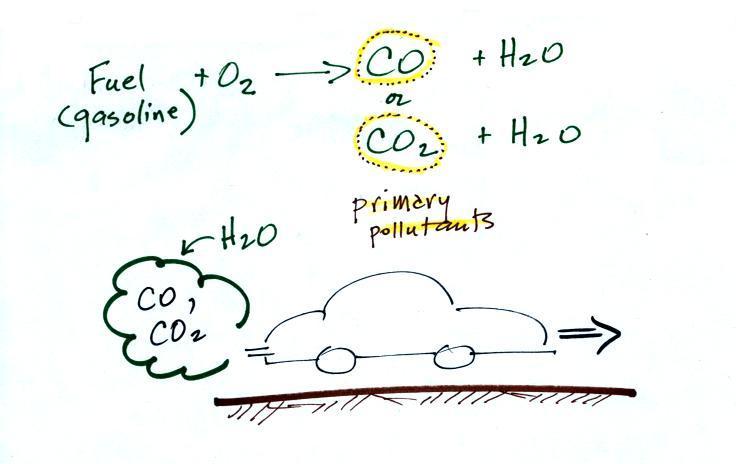
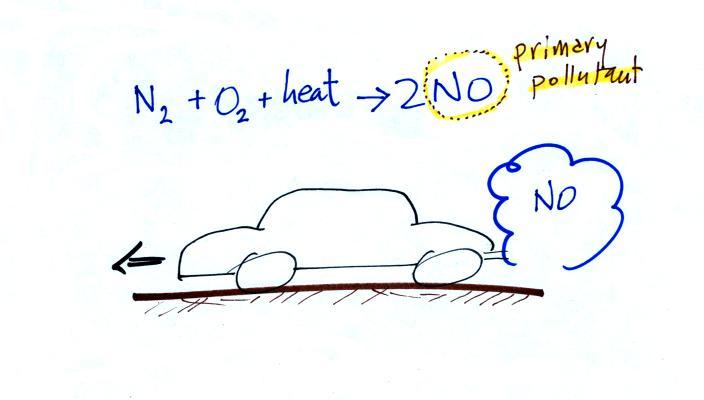
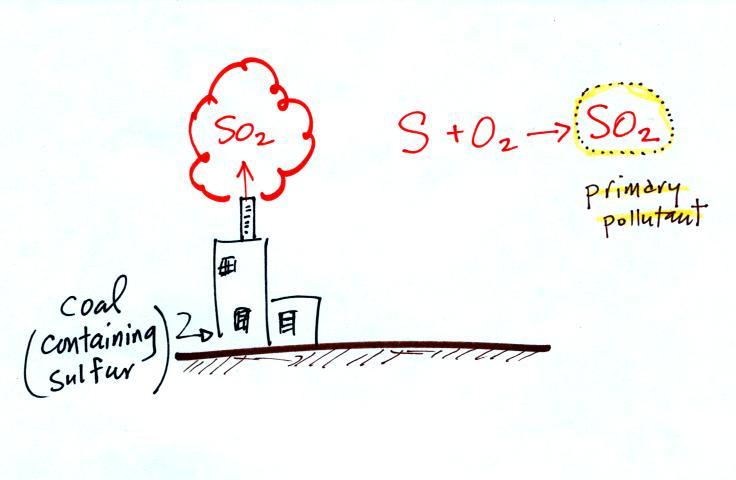
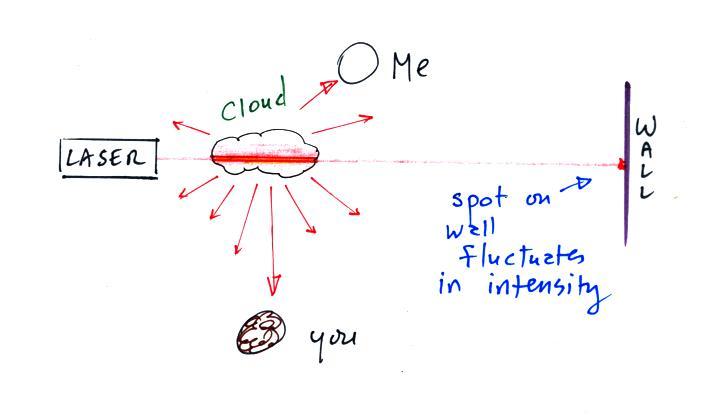
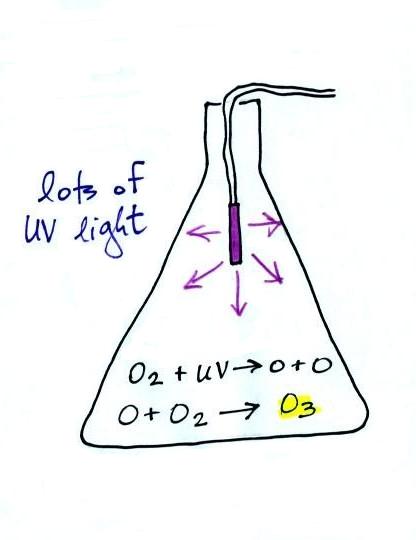
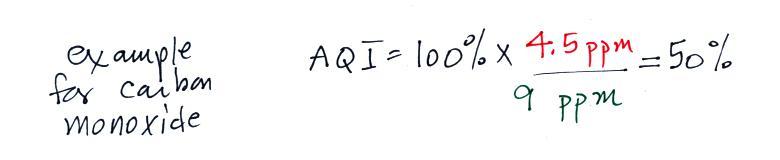The figure above can be found on p. 14a
in the photocopied ClassNotes. The ozone layer (ozone
in the stratosphere) is beneficial, it absorbs dangerous
high energy ultraviolet light (which would otherwise reach
the ground and cause skin cancer, cataracts, etc.
There are some types of UV light that would quite simply
kill us).
Ozone in the troposphere is bad, it is toxic and a
pollutant. Tropospheric ozone is also a key component
of photochemical smog (also known as Los Angeles-type smog)
We'll be making some photochemical smog in a class
demonstration. To do this we'll first need some ozone;
we'll make use of the simple stratospheric recipe (shown
above) for making what we need instead of the more complex
tropospheric process (the 4-step process in the figure
below). You'll find more details a little further down
in the notes.
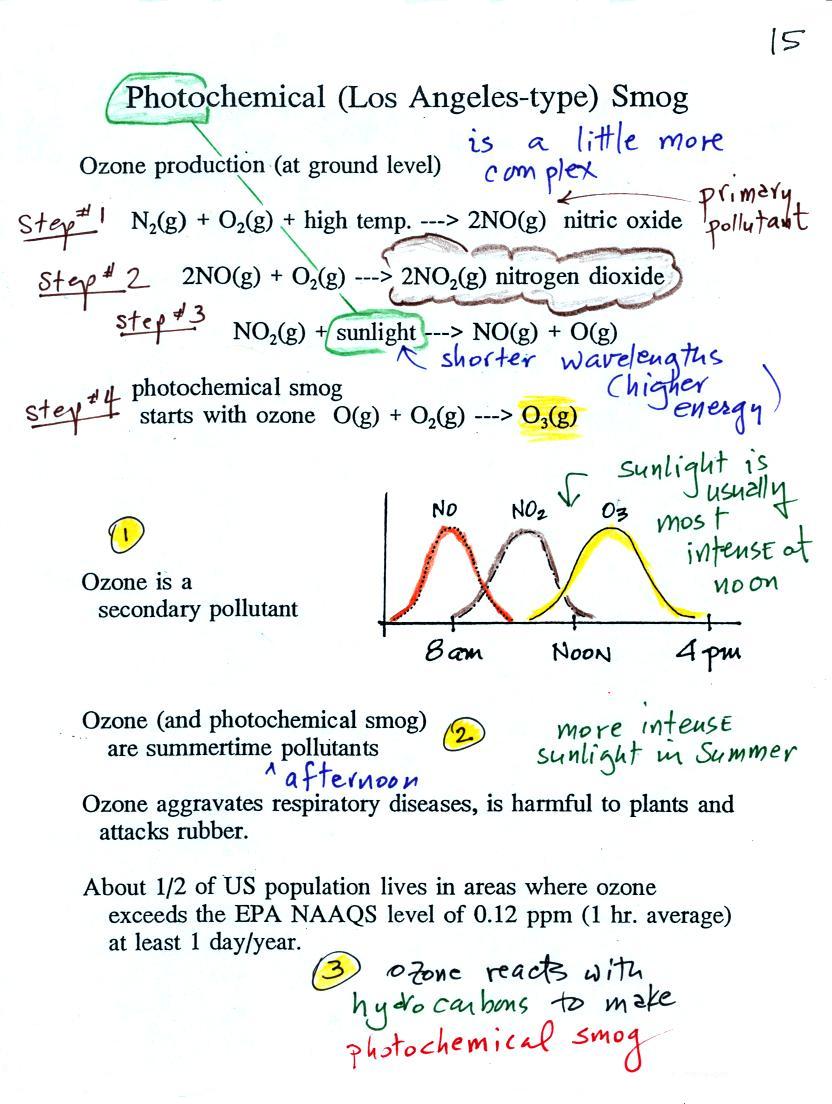
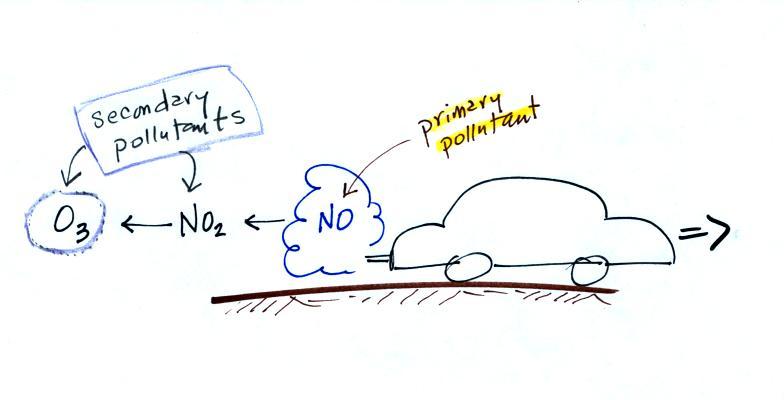
At the top of this figure
(p. 15 in the packet of ClassNotes) you see that a more
complex series of reactions is responsible for the
production of tropospheric ozone. The
production of tropospheric ozone begins with nitric oxide
(NO). NO is produced when nitrogen and oxygen in air
are heated (in an automobile engine for example) and
react.
The NO can then react with oxygen in the air to make
nitrogen dioxide, the poisonous brown-colored
gas that I used to make in class.
Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2 react in a 4th step to
make ozone (O3) just
like happens in the stratosphere. Because ozone does
not come directly from an automobile tailpipe or factory
chimney, but only shows up after a series of reactions in
the air, it is a secondary pollutant. Nitric
oxide (NO) would be the primary pollutant in this example.
NO is produced early in the day (during the morning rush
hour). The concentration of NO2 peaks somewhat later. Because
sunlight is needed in step #3 and because sunlight is
usually most intense at noon, the highest ozone
concentrations are usually found in the afternoon.
Ozone concentrations are also usually higher in the summer
when the sunlight is more intense than at other times of
year.
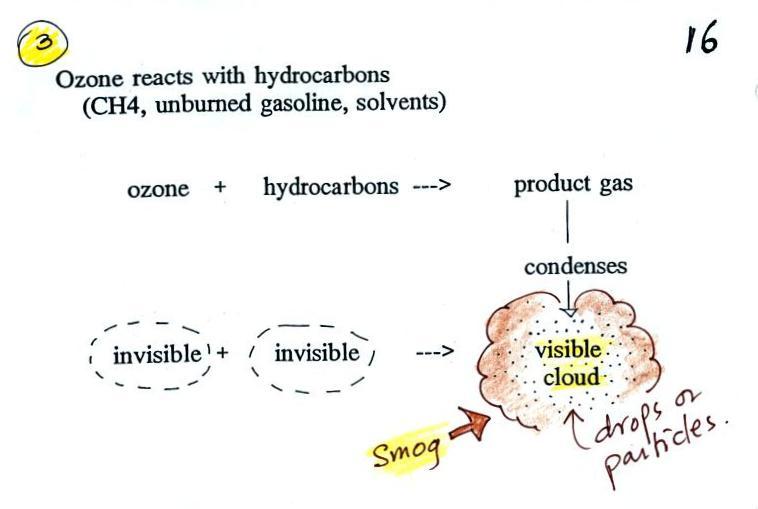
Once ozone is formed, the ozone can react
with a hydrocarbon of some kind to make a product gas.
The ozone, hydrocarbon, and product gas are all invisible,
but the product gas sometimes condenses to make a visible
smog cloud or haze. The cloud is composed of very
small droplets or solid particles. They're too small
to be seen but they are able to scatter light - that's why
you can see the cloud.