Mon., Mar. 31, 2014
Just by accident I happened to catch a story on 60 Minutes last
night about Marcus Roberts who for Wynton Marsalis is "the
greatest American musician most people have never heard of."
Most of the pieces I found on YouTube were too long to play before
class but I did find a couple of short examples of his work.
I think I had time to play "Bolivar Blues"
and "I Got Rhythm"
in class. You really should watch the
60 Minutes Segment though. As a matter of fact, I'm
willing to give you a Green Card if you do. Just write
something (short) down on paper convincing that you really did
watch the video and turn it in before class on Wednesday.
Just to be clear, because there seems to be some confusion, you
need to watch the
60 Minutes Segment in order to earn the Green Card.
The Experiment #2 revised reports have been graded and were
returned in class today. The Experiment #3 reports were due
today also. And next Monday, Apr. 7, is the due date for
everything else: book reports, Scientific Paper reports, and the
Experiment #4 reports.
The Humidity Example Problems Optional Assignment was collected
today also. Here are answers
to the questions on that assignment.
Part 1 of the Quiz #3 Study Guide
is now available online.
Here's an outline of what we'll be covering in class today:
the role that small particles (condensation nuclei)
play in cloud formation
transition from dry haze -> wet haze -> fog
cloud in a bottle demonstration + Mother Nature's
version of the demonstration
clouds and climate change
identifying and naming clouds pt. 1
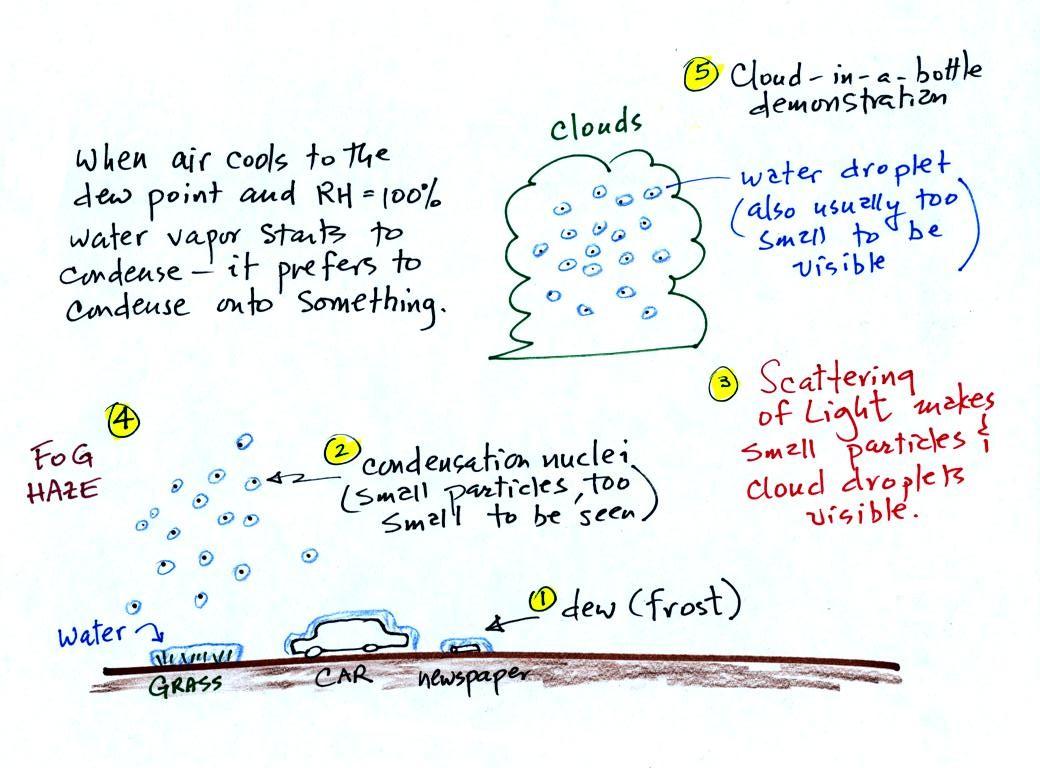
A variety of things
can happen when you cool air to the dew point and the
relative humidity increases to 100%. Point 1
shows that when moist air next to the ground is cooled
to and below the dew point, water vapor condenses onto
(or is deposited onto) the ground or objects on the
ground. This forms dew, frozen dew, and
frost. We had a quick look at this last Friday.
Air above the
ground can also be cooled to the dew point. When
that happens (Point 2 above) it is much easier for
water vapor to condense onto something rather than
just forming a small droplet of pure water.
In air above the ground water vapor
condenses onto small particles in the air called
condensation nuclei. Both the condensation
nuclei and the small water droplets that form on them
are usually too small to be seen with the naked
eye. We can tell they are present (Point 3)
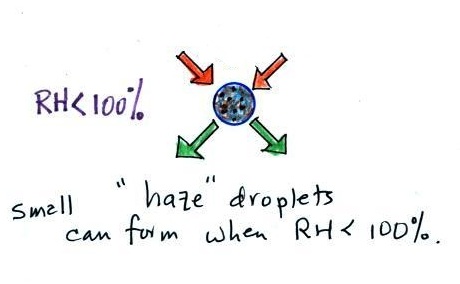
because they scatter sunlight and make the sky
hazy. As humidity increases dry haze turns to
wet haze and eventually to fog (Point 4).
When air well above the ground, clouds can form.
it is much easier for water vapor
to condense onto small particles
called condensation nuclei
|
rather than just
condensing
and forming small droplets
of pure water
|
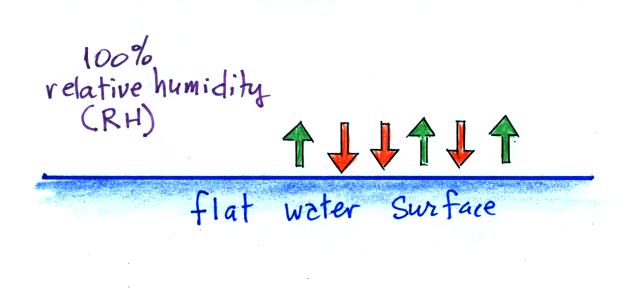
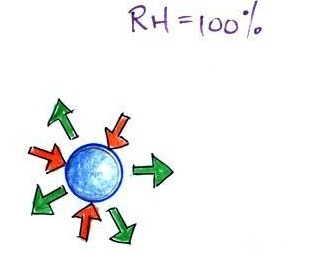
When air is saturated with water vapor (RH = 100%)
We'll first illustrate that when the
air is saturated with water vapor (the relative humidity
is 100%) the rates of evaporation and condensation above a
flat surface of water will be equal. There's
no real reason for picking three arrows each of
evaporation and condensation, the important point is that
they are equal when the RH is 100%.
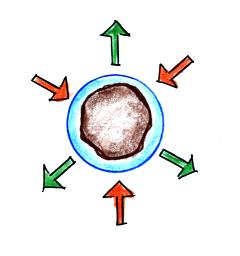
It's hard for water vapor to condense and form a small
droplet of water because small droplets evaporate at a very
high rate. This is known as the curvature effect and is
illustrated below.
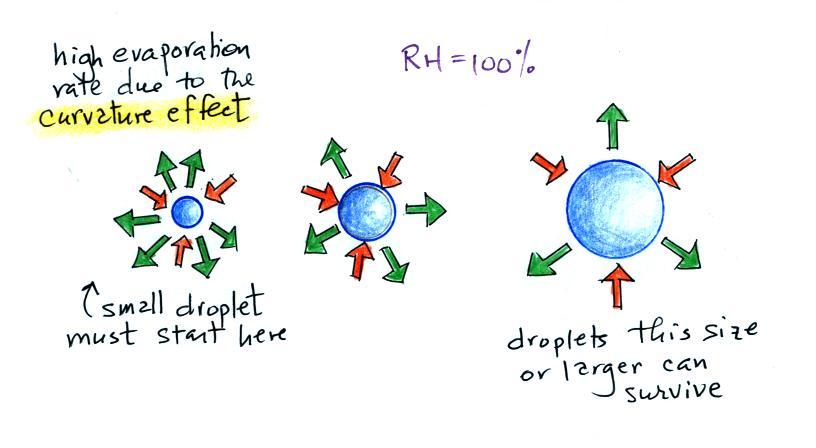
The surface of the smallest droplet above at left has the most
curvature and the highest rate of evaporation (6 arrows). If
a small droplet like this were to form it wouldn't stay around
very long. With it's high rate of evaporation it would
quickly evaporate away and disappear.
The middle droplet is larger and does not evaporate as
quickly.
The drop on the right is large enough that curvature no longer has
an effect. This drop has an evaporation rate (3 arrows) that
is the same as would be found over a flat surface of water.
A droplet like this could survive, but the question is how could
it get this big without going through the smaller sizes with their
high rates of evaporation. A droplet must
somehow reach a critical size before it will be in equilibrium
with its surroundings.
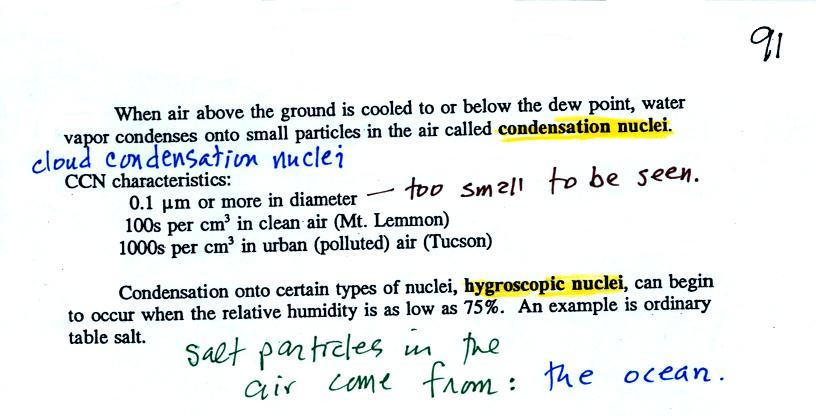
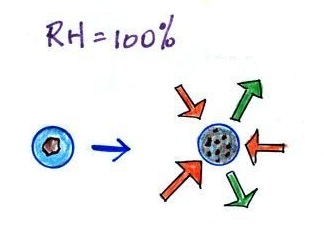
Particles in the air, cloud condensation nuclei (CCN), make it
much easier for cloud droplets to form. The
figure below explains why.
By condensing onto a particle, the water droplet starts out
large enough and with an evaporation rate low enough that it is in
equilibrium with the moist surroundings (equal rates of
condensation and evaporation).
There are always lots of CCN (cloud condensation nuclei in the
air) so this isn't an impediment to cloud formation. The
following information is from p. 91 in the ClassNotes.
Note that condensation onto
certain kinds of condensation nuclei and growth of cloud
droplets can begin even when the relative humidity is below
100%. These are called hygroscopic nuclei. Salt
is an example; small particles of salt mostly come from
evaporating drops of ocean water.
To understand this we first need to learn about the solute
effect (differs a little bit from the
coverage in class)
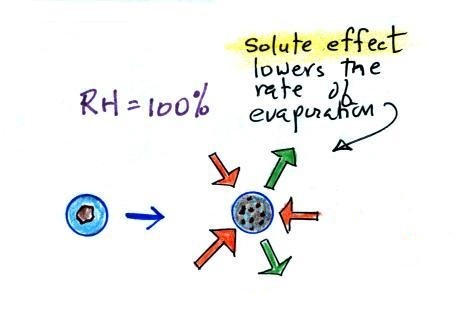
|
|
solution droplet
|
pure water droplet
|
Water vapor condensing onto the
particle in the left figure dissolves the particle. The
resulting solution evaporates at a lower rate (2 arrows of
evaporation). A droplet of pure water of about the same size
would evaporate at a higher rate (4 arrows in the figure at
right). Note the rates of condensation are equal in both
figures above, the RH is 100% in both figures above and the rates
of condensation are equal.
The next figure compares solution droplets that form when the
RH is 100% (left figure) and when the RH is less than 100%.
The solution droplet will grow in the RH=100% environment at
left. You can tell the RH is less than 100% in the figure at
right because there are now only 2 arrows of evaporation.
But because the solution droplet only has 2 arrows of evaporation
it can form and be in equilibrium in this environment.
Don't worry too much about all the details. The key point is
that particles help clouds to form.
The following figure is at the bottom of p. 91 in the
ClassNotes.
This figure shows how cloud condensation nuclei and
increasing relative humidity can affect the appearance of the sky
and the visibility.
The air in the left most figure is relatively dry. Even
though the condensation nuclei particles are too small to be seen
with the human eye you can tell they are there because they
scatter sunlight. When you look at the sky you see the deep
blue color caused by scattering of sunlight by air molecules mixed
together with some white sunlight scattered by the condensation
nuclei. This changes the color of the sky from a deep blue
to a bluish white color. The more particles there are the
whiter the sky becomes. This is called "dry haze."
Visibility under these conditions might be a few tens of miles.
The recent pollution problems in Paris is most likely an
extreme case of dry haze. Much like the severe pollution
events in China discussed earlier in the semester. Cars with even
numbered license plates weren't allowed into the city on Monday,
odd numbers were banned on Tuesday. Public transportation
was free for a short time to try to reduce automobile use.
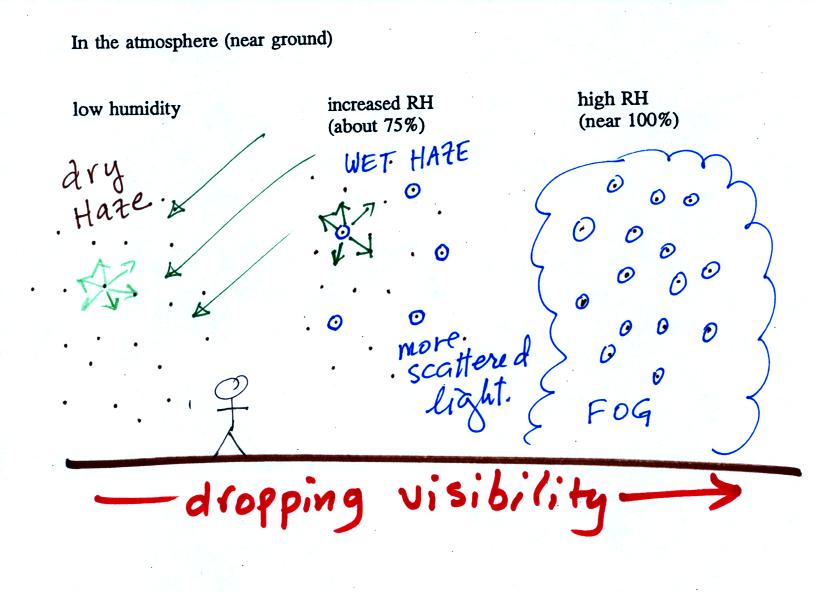
The middle picture shows what happens when you drive from the
dry southwestern part of the US into the humid southeastern US or
the Gulf Coast. One of the first things you would notice is
the hazier appearance of the air and a decrease in
visibility. Because the relative humidity is high, water
vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small
water droplets. The water droplets scatter more sunlight
than just small particles alone. The increase in the amount
of scattered light is what gives the air its hazier appearance.
This is called "wet haze." Visibility now might now only be
a few miles.

|

|
Thin fog
(perhaps even wet haze)
with pretty good visibility
(source
of the image)
|
Thick fog
(visibility was less than 500 feet)
(source
of the image)
|
Finally when the relative humidity increases to 100% fog
forms. Fog can cause a severe drop in the visibility.
The thickest fog forms in dirty air that contains lots of
condensation nuclei. That is part of the reason the Great
London Smog of 1952 was so impressive. Visibility was at
times just a few feet!
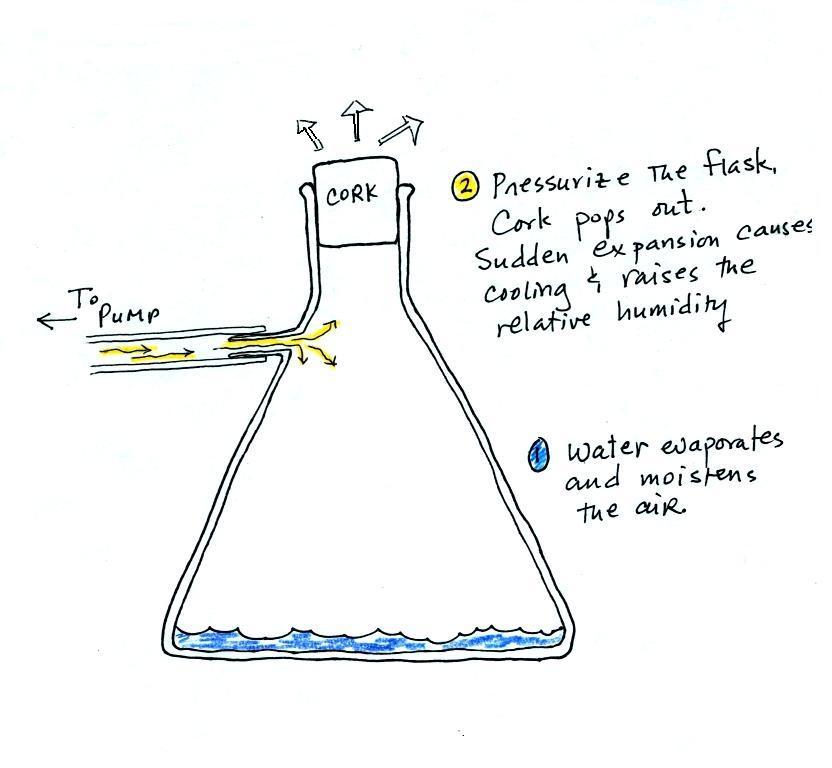
Next up was the cloud-in-a-bottle demonstration. Cooling
air, changing relative humidity, condensation nuclei, and
scattering of light are all involved in this demonstration.
We used a strong, thick-walled, 4
liter vacuum flask (designed to not implode when all of the
air is pumped out of them, they really aren't designed to be
pressurized). There was a little water in the bottom of
the flask to moisten the air in the flask. Next we
pressurized the air in the flask with a bicycle pump. At
some point the pressure blows the cork out of the top of the
flask. The air in the flask expands outward and
cools. This sudden cooling increases the relative
humidity of the moist air in the flask to 100% ( probably more
than 100% momentarily ) and water vapor condenses onto cloud
condensation nuclei in the air. A very faint cloud
became visible at this point. Believe it or not that's
the way I like the demonstration to work.
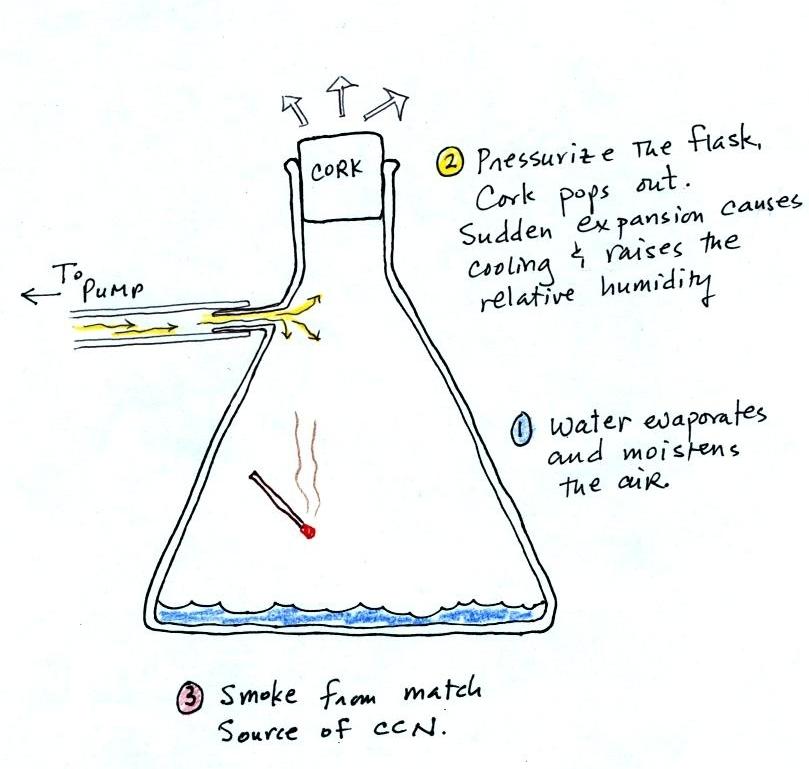
The demonstration was repeated an additional time with one
small change. A burning match was dropped into the
bottle. The smoke from the matches added lots of very small
particles, condensation nuclei, to the air in the flask. The
same amount of water vapor was available for cloud formation but
the cloud that formed this time was quite a bit "thicker" and much
easier to see. To be honest the burning match probably also
added a little water vapor (water vapor together with carbon
dioxide is one of the by products of combustion).
This effect has some implications for climate change.
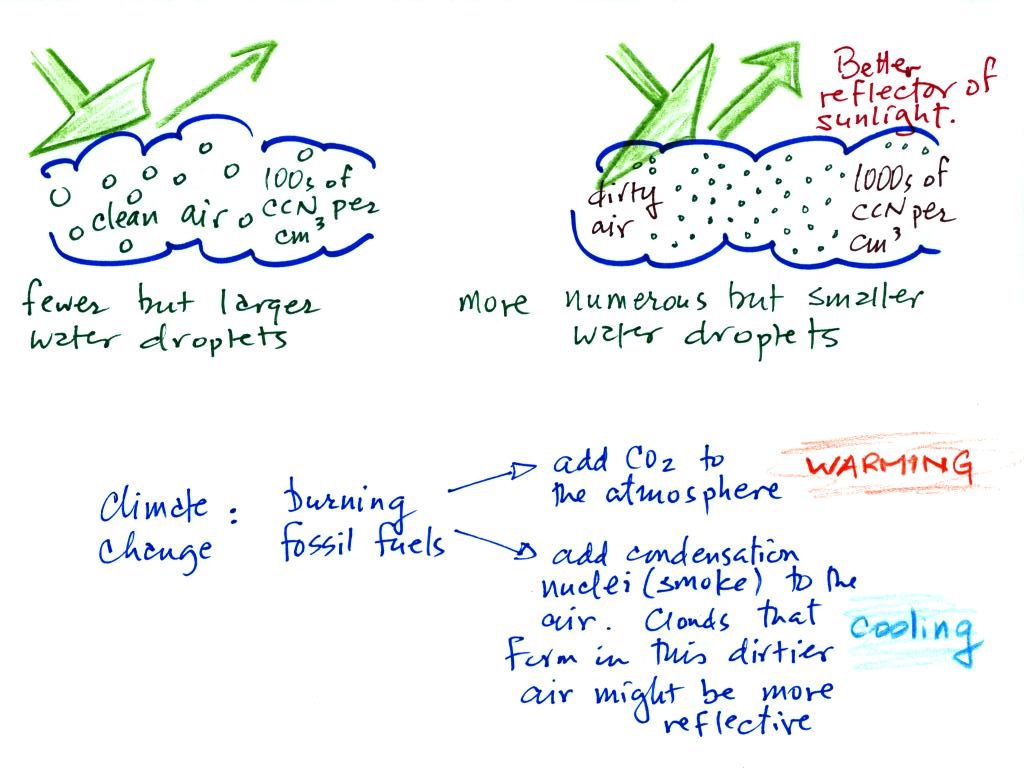
A cloud that forms in dirty air is composed of a large number
of small droplets (right figure above). This cloud is more
reflective than a cloud that forms in clean air, that is
composed of a smaller number of larger droplets (left
figure).
Combustion of fossil fuels adds carbon dioxide to the
atmosphere. There is concern that increasing carbon
dioxide concentrations (and other greenhouse gases) will enhance
the greenhouse effect and cause global warming. Combustion
also adds condensation nuclei to the atmosphere (just like the
burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form,
might make the clouds more reflective, and might cause
cooling. There is still quite a bit of uncertainty about
how clouds might change and how this might affect climate.
Remember that clouds are good absorbers of IR radiation and also
emit IR radiation.
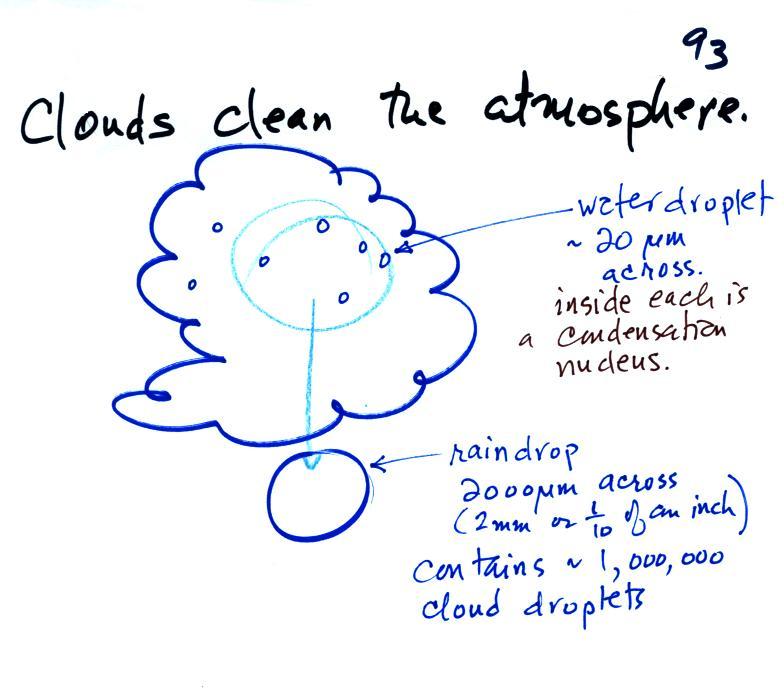
Clouds are one of the best ways of cleaning the atmosphere
A cloud is composed of small water droplets (diameters of 10 or 20
micrometers) that form on particles ( diameters of perhaps 0.1 or
0.2 micrometers). The droplets "clump" together to form a raindrop
(diameters of 1000 or 2000 micrometers which is 1 or 2
millimeters), and the raindrop carries the particles to the
ground. A typical raindrop can contain 1 million cloud
droplets so a single raindrop can remove a lot of particles from
the air. You may have noticed how clear the air seems the
day after a rainstorm; distant mountains are crystal clear and the
sky has a deep blue color. Gaseous pollutants can dissolve
in the water droplets and be carried to the ground by rainfall
also. We'll be looking at the formation of precipitation
later this week.
And here's Mother Nature's version of the cloud in a bottle
demonstration.
A brush fire in this picture is
heating up air and causing it to rise. Combustion also
adds some moisture and lots of smoke particles to the
air. You can see that initially the rising air doesn't
form a cloud. A little higher and once the rising air
has cooled enough (to the dew point) a cloud does form.
And notice the cloud's appearance - puffy and not a layer
cloud. Cumulo or cumulus should be in the cloud
name. These kinds of fire caused clouds are called
pyrocumulus clouds. The example above is from a
Wikipedia article about these kinds of clouds.
The
fire in this case was the "Station Fire" burning near Los
Angeles in August 2009.
We'll be spending the whole class on Wednesday learning to
identify and name clouds.
I got an early start on that at the end of class today.
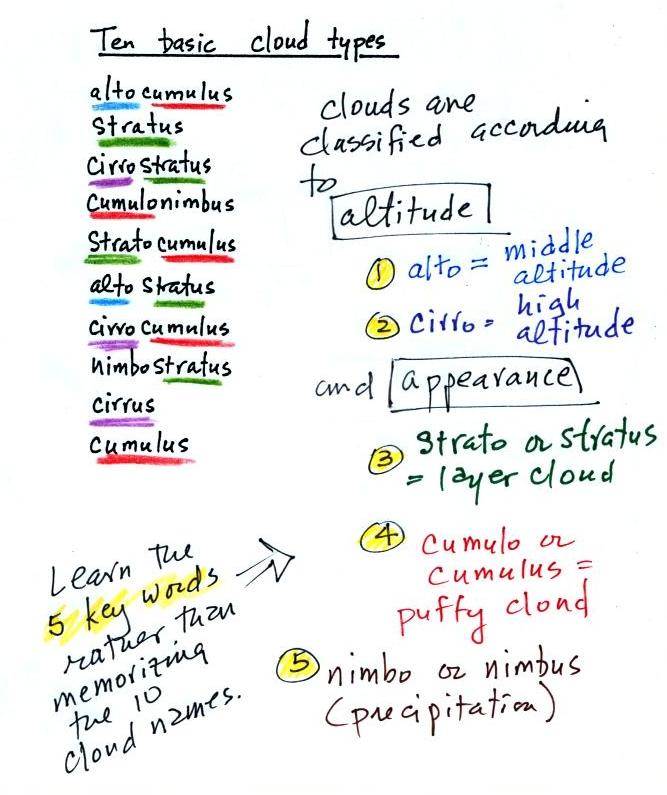
The ten main cloud types are listed
below (you'll find this list on p. 95 in the ClassNotes)
I'm hoping you'll try to learn these 10
cloud names. There is a smart and a not-so-smart way of
learning these names. The not-so-smart way is to just
memorize them. Because they all sound alike you will
inevitably get them mixed up. And I'm hoping you'll be
able to sketch each of the clouds and describe them in
words. That gets to be a lot of material to try to just
memorize.
A better way is to recognize that all
the cloud names are made up of key words. Clouds are
classified using just two criteria: altitude and
appearance. There are 4 key words that tell you
something about the cloud's altitude and appearance (and a 5th
key word for clouds that are producing precipitation wasn't
mentioned in class). My recommendation is to learn the
key words and what they mean. Then you
can usually construct a cloud name by taking key words from both
the altitude and appearance groups and combining them.
Here are some
examples to start to get a feel for how this works.
Examples of puffy patchy (cumuliform)
clouds found at different altitudes
 |
high altitude cloud
the patches of cloud are small because they are far
away
a cirrocumulus cloud
cirro means high altitude
cumulus means patchy
|
 |
middle altitude cloud
the patches of cloud are bigger because they closer to the
ground
an altocumulus cloud
|
 |
low altitude cloud
cumulus clouds
(there is no key word for low altitude)
|
Examples of clouds of different appearance
 |
 |
 |
featureless Stratiform
cloud
(layer cloud)
Strato- or -stratus will be part of the cloud name
an altostratus cloud
|
this in
between case,
a "lumpy layer cloud" combines features of both
stratiform & cumuliform clouds
and is named stratocumulus
|
patchy, puffy Cumuliform
cloud
cumulo- or -cumulus will be part of the cloud name
this is the same cumulus cloud
that was shown above
|