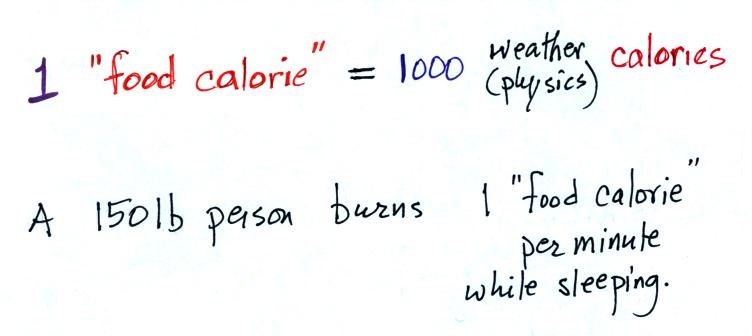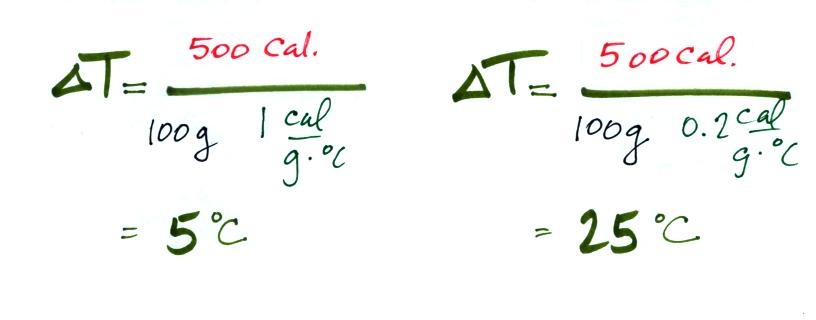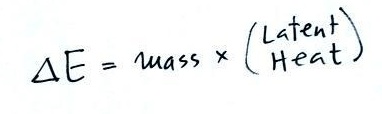Friday Feb. 21, 2014
Xavier Rudd "Better
People", "Time to
Smile" before class because I'm feeling a lot better after
having graded 80+ experiment reports and (together with my TAs)
having looked at 280+ quizzes.
Quiz #1 has been graded and was returned in class
today. The average score was 75%. The
calculation below shows what sort of final grade you might
expect if you were to score 76% on the remaining quizzes (the
76% was the average grade for the other section of the class and
I didn't want to redo the figure, the end result would be nearly
the same if 75% were used in the calculation).
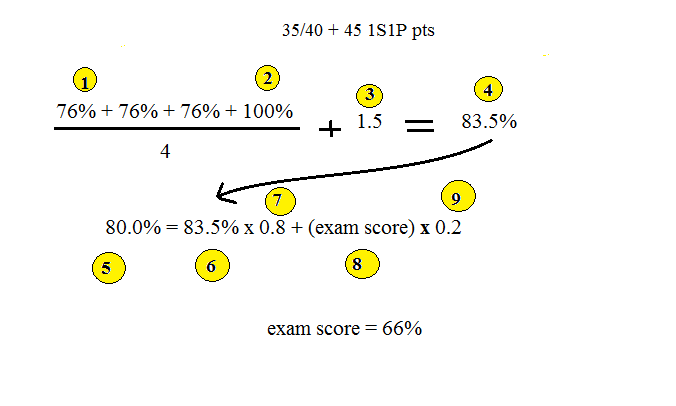
We'll assume you're happy with a B in the class (especially
since you got Cs on all the quizzes). We calculate in the
2nd equation how low of a score you could get on the Final Exam
and still preserve the B. The 80% at pt. 5 shows the
desired grade. Your current average (pt. 6) is 80% (pt. 7)
of your overall grade. Your final exam score (pt. 8) will
count as 20% of your overall grade (pt. 9).
You'd only need 66% on the Final Exam to keep a B in the class
(69% if the 75% average were used throughout).
How is it possible that with Cs on the quizzes and a D on the
Final Exam you would end up with a B in the class? The
answer is the writing grade. There is no reason not to get
100% on the writing grade and doing so can have a big effect on
your overall grade.
This is about as much math as you'll ever have to do in this
class.
An In-class Optional Assignment
was collected at the end of today's class. Students
provided answers to 5 questions asked during the course of the
class. If you weren't in class but are reading these
online notes you can turn in answers to the question embedded in
the notes and receive at least partial credit.
We're done with weather maps for the time being. Though
if interesting weather appears imminent I'll try to mention it
in class.
If we were using a textbook in this class we'd be moving
into Chapter 2! During the next couple of weeks we will be
concerned with energy, temperature, heat, energy transport, and
energy balance between the earth, atmosphere, and space.
It is easy to lose sight of the main concepts because there
are so many details. The following is an introduction to
this new section of material and most of the figures are found
on pages 43 & 44 in the photocopied ClassNotes.
Types of energy
We will learn the names of several different types or forms
of energy.
Kinetic energy is energy of motion. Some examples (both
large and microscopic scale) are mentioned and sketched
above. This is a relatively easy to visualize and
understand form of energy.
Radiant energy is a very important form of energy that was
for some reason left off the original list in the ClassNotes
(pps 43&44). Electromagnetic radiation is
another name for radiant energy. Sunlight is an example of
radiant energy that we can see and feel (you feel warm when you
stand in sunlight). Everyone in the classroom is emitting
infrared light, an invisible form of radiant energy. And
actually the walls, ceiling, floor and even the air in the
classroom are also emitting infrared light.
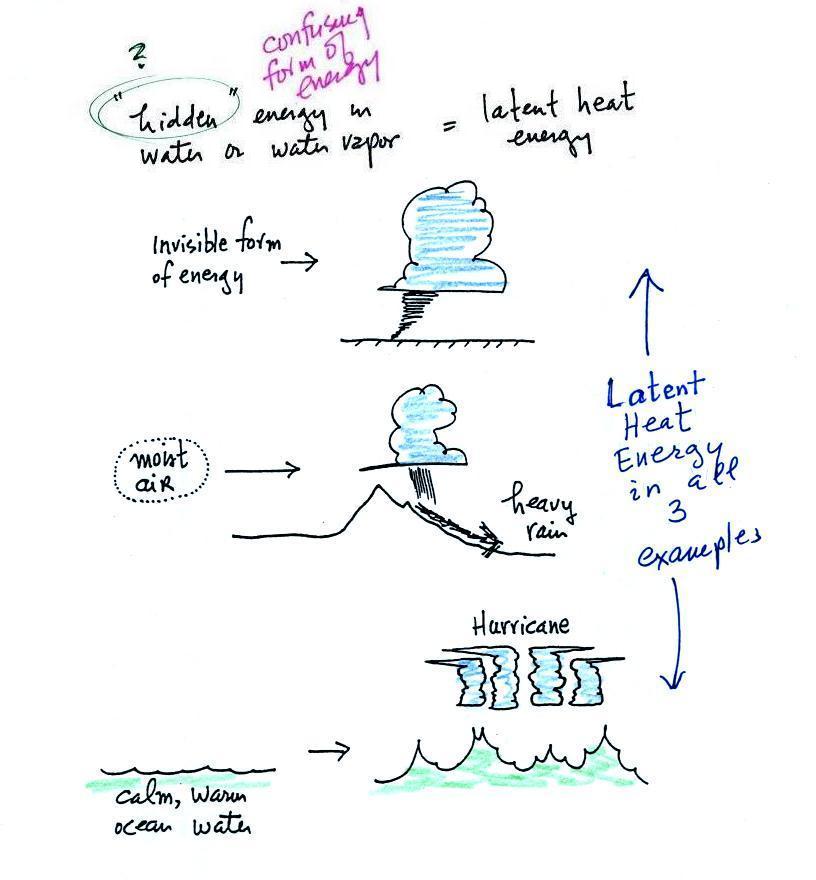
Latent heat energy is an
under-appreciated and rather confusing type of energy. The
word latent refers to energy that is hidden. That's
part of the problem. But it is also the fact that
the energy is contained in water vapor and water.
That seems like an unlikely place for energy to be
found. The hidden energy emerges when
water vapor condenses or water freezes (the energy had
been added earlier when ice was melted or water was
evaporated).
Here's the 1st of the In-class Optional Assignment
questions:
1. Can you think of any additional types/forms of
energy?
We'll usually be using calories as units of
energy. 1 calorie is the energy need to warm 1
gram of water 1 C (there are about 5 grams of water in
a teaspoon)
Here's a little miscellaneous information that you
don't need to worry about remembering. You've
probably seen the caloric content of food on food packages
or on menus in restaurants. 1 food calorie is
actually 1000 of the calories mentioned above.
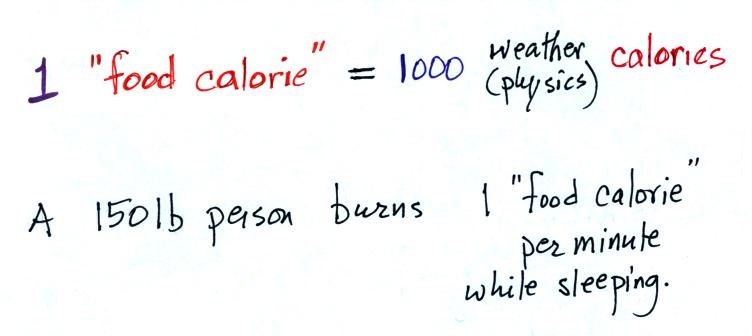
A 150 pound person would burn
almost 500 calories while sleeping during the night (8
hours x 60 minutes per hour x 1 food calorie per
minute). This is about the energy contained in a
donut.
Here's another In-class Optional Assignment question
2. What's the difference between energy &
power?
Energy
transport
Four energy transport processes are listed
below
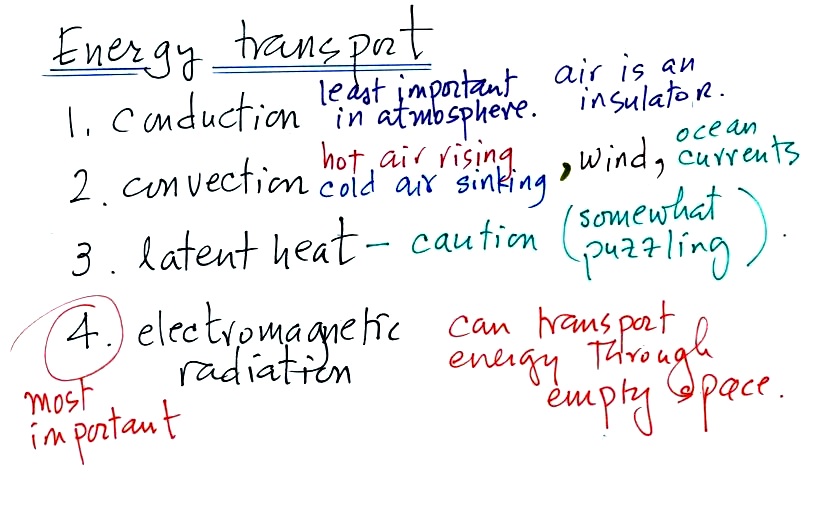
By far the most important
process is at the bottom of the list above. Energy
transport in the form of electromagnetic radiation (sunlight for
example) is the only process that can transport energy through
empty space. Electromagnetic radiation travels both to the
earth (from the sun) and away from the earth back into
space. Electromagnetic radiation is also responsible for
about 80% of the energy transported between the
ground and atmosphere.
You might be surprised to learn that latent heat is the
second most important transport process. This term latent
heat can refer to both a type of energy and an energy transport
process.
Rising parcels of warm air and sinking parcels of cold air
are examples of free convection. Because of convection you
feel colder or a cold windy day than on a cold calm day (the
wind chill effect). Ocean currents are also an
example of convection.
Convection is a 3rd way of causing rising air motions in the
atmosphere (convergence into centers of low pressure and fronts
are two other ways we've encountered so far)
Conduction is the least important energy transport at least
in the atmosphere. Air is such a poor conductor of energy
that it is generally considered to be an insulator.
The next In-class Optional Assignment question is
3. The Gulf Stream is a well known ocean
current. Where is it? What do you know about
it? Is it a warm or cold current? What direction
does it move? Can you see or appreciate how it is able
to transport energy?
Energy balance and the atmospheric greenhouse effect
The next picture (the figure in the ClassNotes has been split
into three parts for improved clarity) shows energy being
transported from the sun to the earth in the form of
electromagnetic radiation.
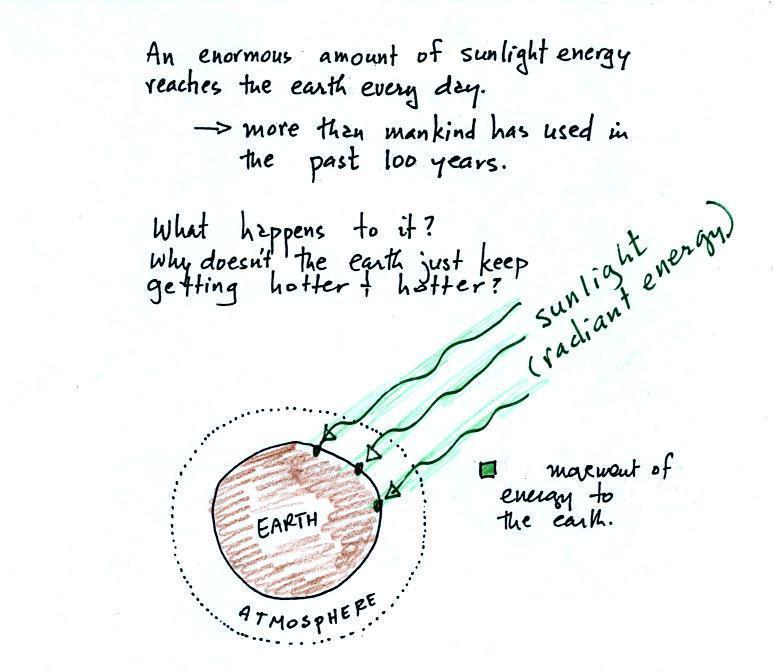
We are aware of this energy
because we can see it (sunlight also contains invisible
forms of light) and feel it. With all of this
energy arriving at and being absorbed by the earth, what
keeps the earth from getting hotter and hotter? If
you park your car in the sun it will heat up. But
there is a limit to how hot it will get. Why is
that?
It might be helpful
when talking about energy balance to think of a bank
account. If you periodically deposit money into your
account why doesn't the balance just grow without
limit. The answer is that you also take money out of
the account and spend it. The same is true of energy
and the earth. The earth absorbs incoming sunlight
energy but also emits energy back into space (the orange
and pink arrows in the figure below). Energy is
emitted by both the surface of the earth and the
atmosphere.
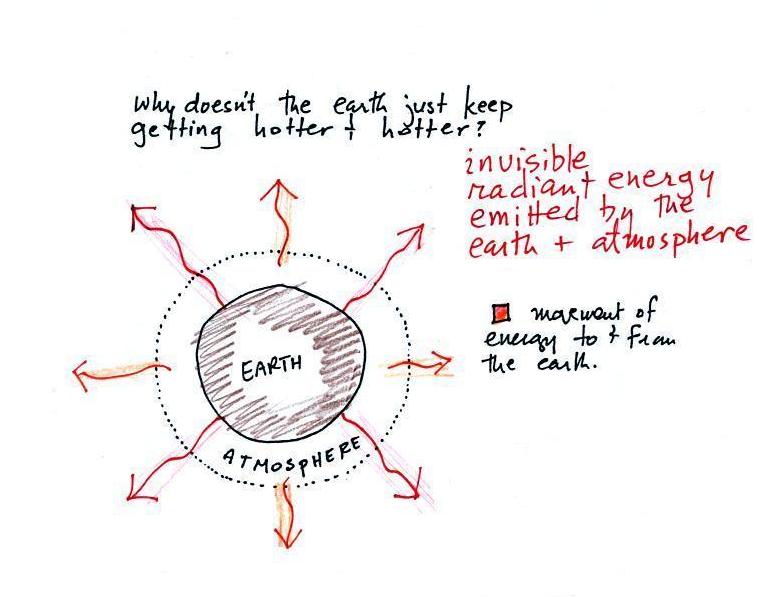
Energy emitted in the form of infrared light is an invisible
form of energy (it is weak enough that we don't usually feel it
either). A balance between incoming and outgoing energy is
achieved and the earth's annual average temperature remains
constant.
We will also look closely at energy transport between the
earth's surface and the atmosphere (see the figure below). This
is where latent heat energy transport, convection and conduction
operate (they can't transport energy beyond the atmosphere and
into outer space).
That is also where the atmospheric greenhouse
functions. That will be a important goal - to better
understand how the atmospheric greenhouse effect works.
The next to last question on today's assignment pertains to
the figure above.
4. How is energy transported back and forth between
the earth's surface and atmosphere?
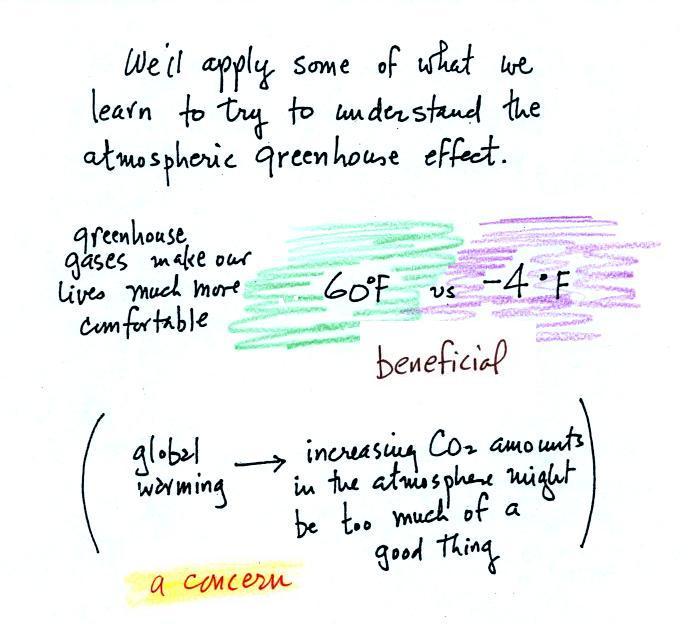
The detrimental side is that atmospheric greenhouse gas
concentrations are increasing (no real debate about that).
This might enhance or strengthen the greenhouse effect and cause
the earth to warm (some debate here particularly about how much
warming there might be). While that doesn't necessarily
sound bad it could have many unpleasant side effects (lots of
debate and uncertainty about this also). That's a subject
we'll explore later in the semester.
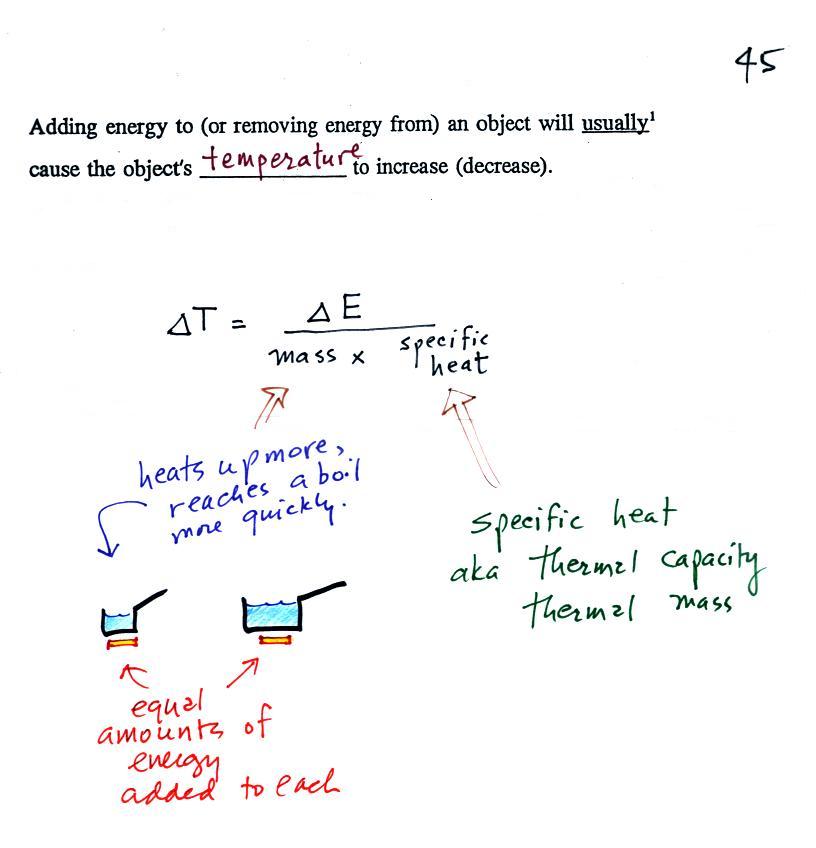
When you add energy to an object, the object will usually warm
up (or if you take energy from an object the object will
cool). It is relatively easy to come up with an equation
that allows you to figure out what the temperature change will be
(this is another equation I'll write on the board before the
next quiz if you ask me to - try to understand it, you don't have
to memorize it).
The temperature change, ΔT, will first depend on how much
energy was added, ΔE. This is a
direct proportionality, so ΔE is in the
numerator of the equation (ΔE and ΔT
are both positive when energy is added, negative when energy is
removed)
When you add equal amounts of energy to large and small
pans of water, the small pan will heat up more quickly. The
temperature change, ΔT, will depend on the
amount of water, the mass. A small mass will mean a large ΔT,
so mass should go in the denominator of the equation.
Specific heat is what we use to account for the fact that
different materials react differently when energy is added to
them. A material with a large specific heat will warm more
slowly than a material with a small specific heat. Specific
heat has the same kind of effect on ΔT as
mass. Specific heat is sometimes called "thermal mass" or
"thermal capacity." You can think of specific
heat as being thermal inertia - a substance with high specific
heat, lots of thermal inertia, will be reluctant to change
temperature.
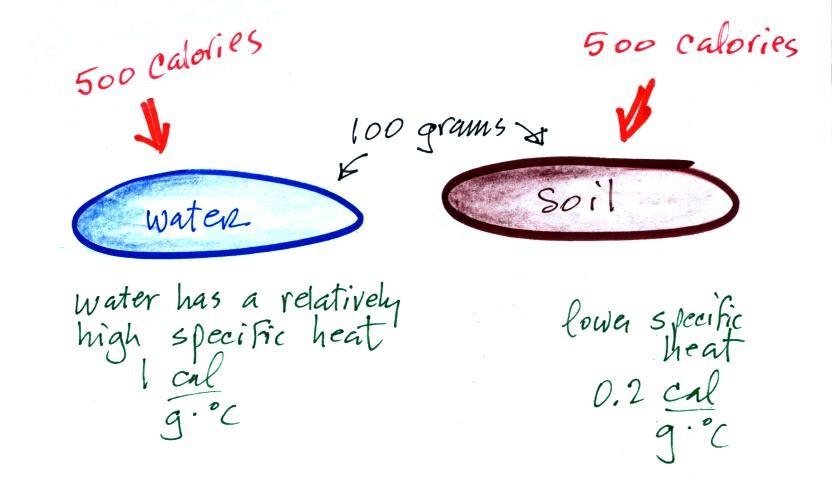
Here's an important example that will show the effect of
specific heat (middle of p. 45).
Equal amounts of energy (500 calories) are added to
equal masses (100 grams) of water and soil. We use water and
soil in the example because most of the earth's surface is either
ocean or land. Before we do the calculation, try to guess which
material will warm up the most. Everything is the same
except for the specific heats. Will water with its 5 times
larger specific heat warm up more or less than the soil?
The details of the calculation are shown below.
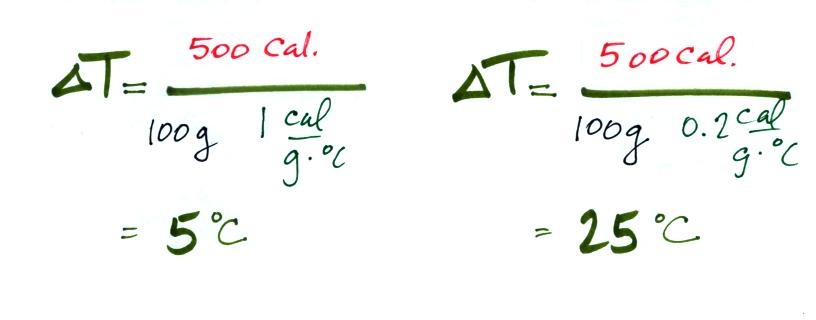
With its higher specific heat, the water
doesn't heat up nearly as much as the soil. If we had
been removing energy the wouldn't cool off as much as the soil
would.
These different rates of warming of water and soil have
important effects on regional climate.
Oceans moderate the climate. Cities near a large body of
water won't warm as much in the summer and won't cool as much
during the winter compared to a city that is surrounded by land.
Water's ΔT is smaller than land's because water has
higher specific heat.
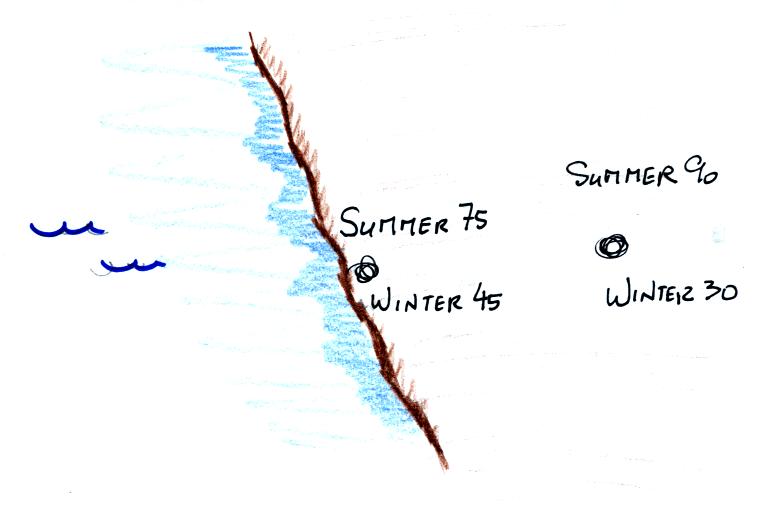
The yearly high and low monthly average temperatures are shown
at two locations above. The city on the coast has a 30o
F annual range of temperature (range is the difference
between the summer and winter temperatures). The city
further inland (assumed to be at the same latitude and altitude)
has an annual range of 60o F. Note that
both cities have the same 60o F annual average
temperature.
Here's another situation where you can take advantage of
water's high specific heat to moderate climate on a smaller scale.
This is what I'll be
doing about this weekend, planting tomatoes. I
do this so that they can start to make tomatoes before
it starts to get too hot in May. In February it
can still get cold enough to kill tomatoes so they need
some protection (the broccoli and lettuce in
the background can handle a light frost).
Here's one way of doing that. You can surround
each plant with a "wall o water" - a teepee like
arrangement that surrounds each plant. The cylinders are
filled with water and they take advantage of the high
specific heat of water and won't cool as much as the air or soil
would during a cold night. The walls of water produce a warm
moist micro climate that the tomato seedlings love. The
plastic is transparent so plenty of sunlight can get through.
Now in anticipation of an in-class experiment planned
for next Monday.
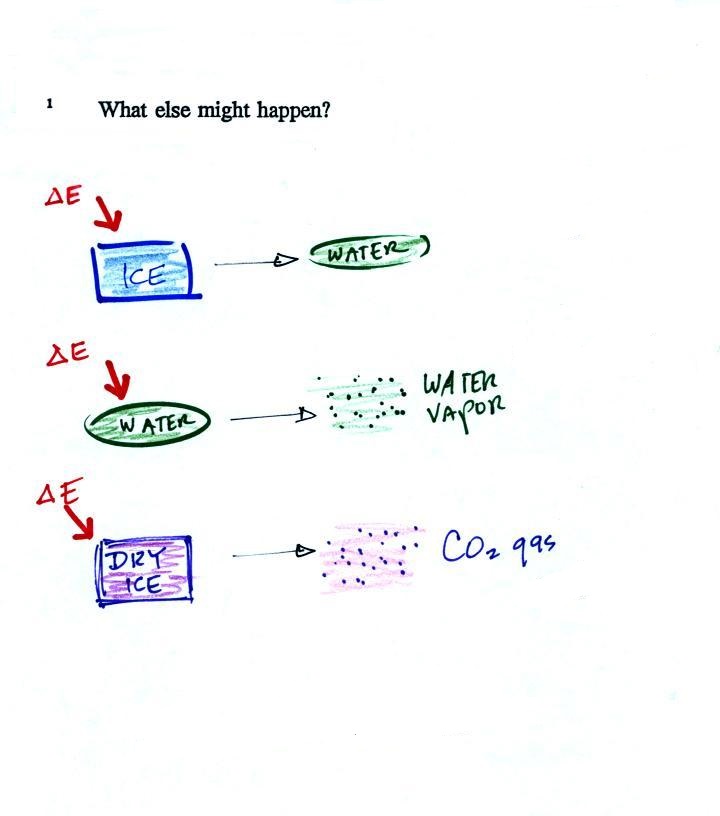
Adding energy to something will usually cause its temperature
to change. But not always. What else could happen?
Adding energy to ice will first cause it to warm to 0 C.
But then it will stop warming and will start to turn to
water. Adding energy to an object can cause the object or
material to change phase. It is very easy
to calculate how much energy is needed to cause a phase change.
The energy needed depends on the amount of material present
(the mass) and on the material itself (that's the Latent Heat term
above). It also depends on the specific phase change.
I.e. there are different Latent Heat values depending on whether
the material changes from solid to liquid, liquid to gas, or solid
to gas.
Here's the last of the In-class Optional Assignment Questions
5. Can you name the three phase changes shown in the
figure above.