


 The summit of Mauna Loa is the dark area to the left of center on this image of the "big island" of Hawaii. (source of this image) |
 |
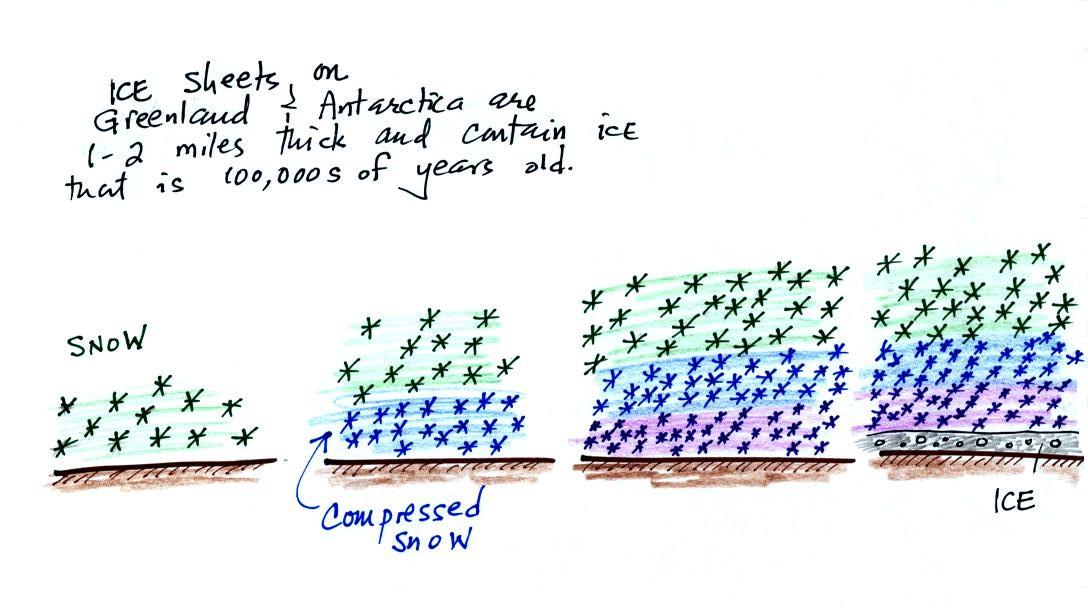

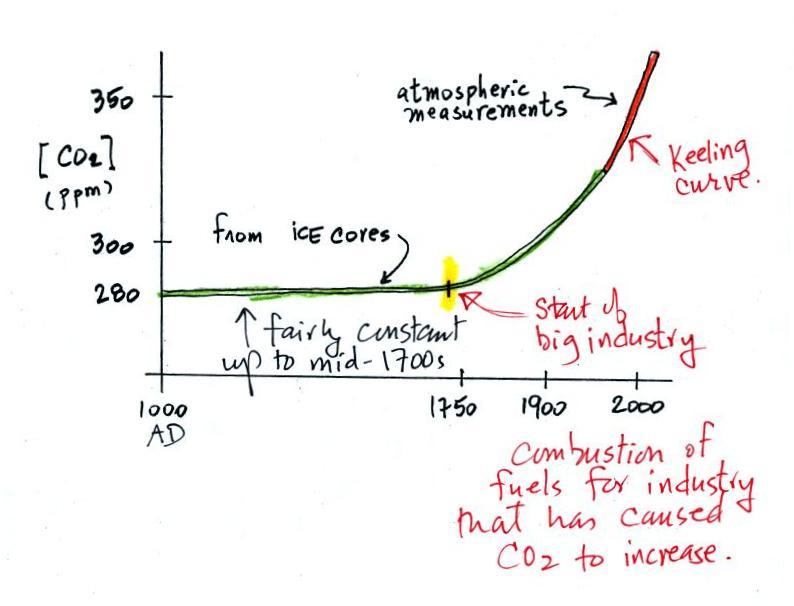
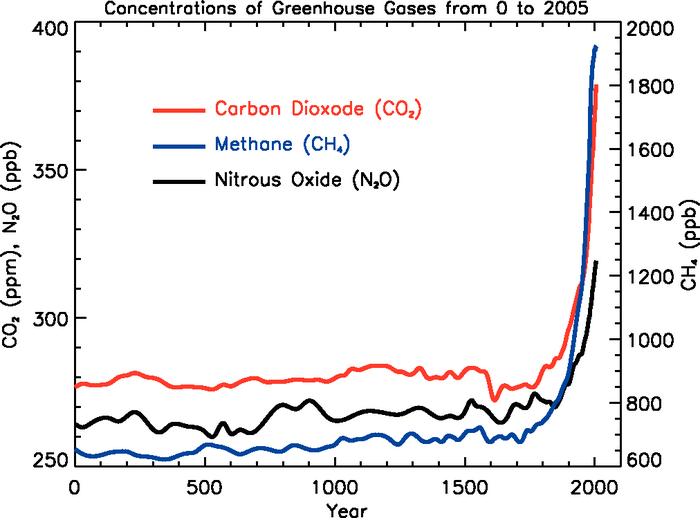
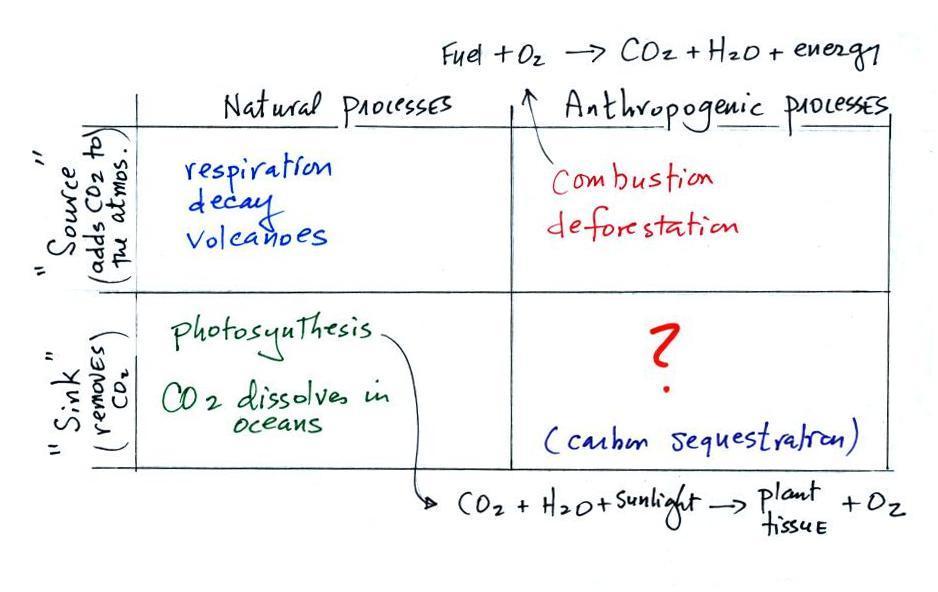
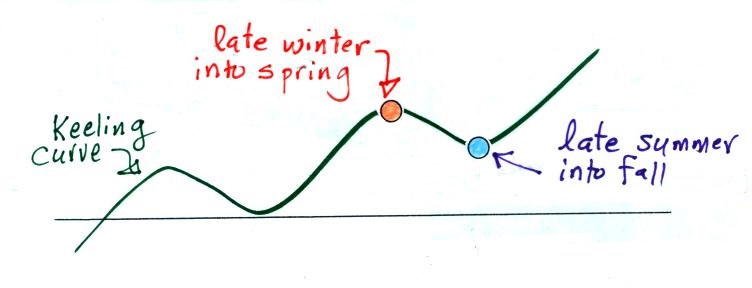
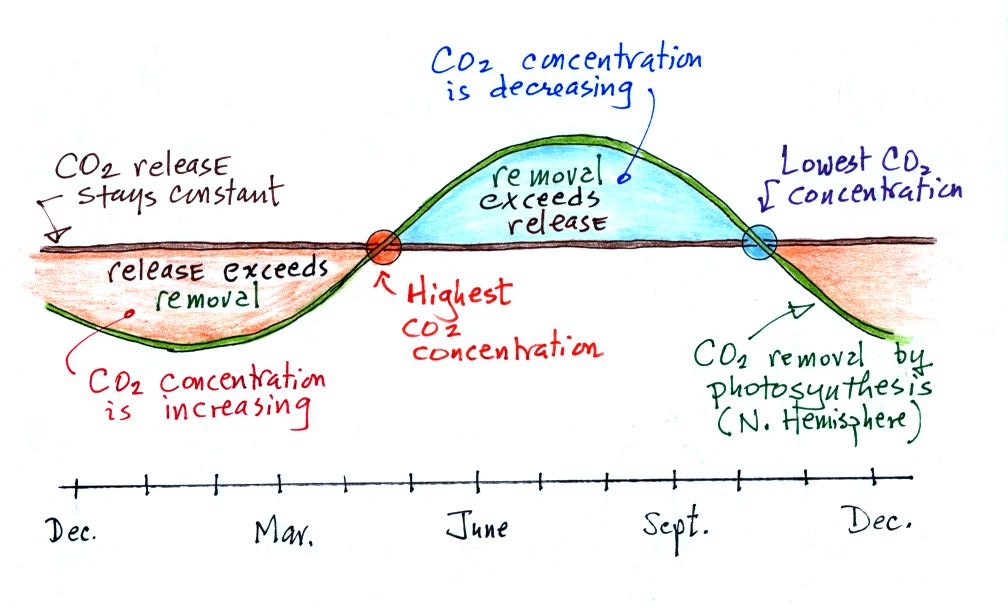
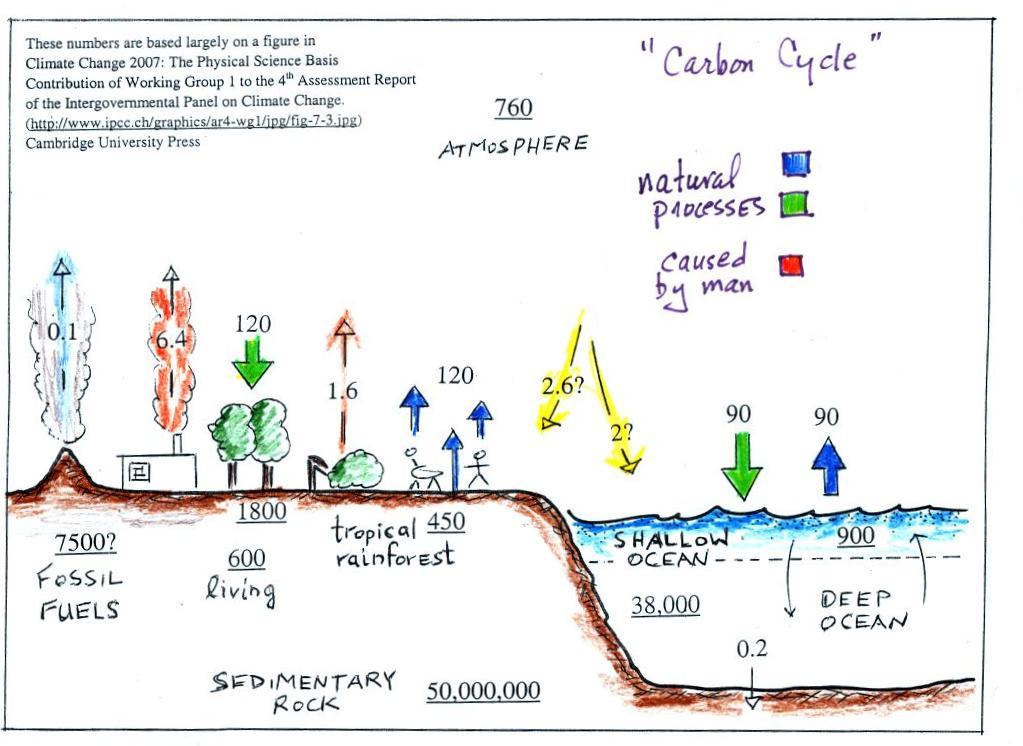
| concentration |
physiological symptoms |
| 1% (10,000 ppm) |
some people start to experience
drowsiness. |
| 2% |
mildly narcotic, increases blood
pressure and pulse rate, and decreases hearing |
| 5% |
shortness of breath, dizziness,
confusion, anxiety, headache |
| 8% |
dimmed sight, sweating, muscular
tremors, loss of consciousness after 5 to 10 minutes
exposure |
 |
 |
| The site as it appears
now (source
of this photograph) |
The site as it might have
appeared in ancient times. This photograph,
credited to Francesco D'Andria, the Italian archaeologist
that announced the discovery in March, 2013, is found in a news
report from the National Geographic Society. |