Monday Feb. 20, 2012
click here to
download today's notes in a more printer friendly format
Time for only one Alison Krauss song. I think you heard "Sister
Rosetta Goes Before Us", though it might have been "Dimming of the Day"
A reminder that there are two assignments that you can turn in
this week if you want to (neither of them is required). The
first,
a
Surface weather map analysis, is
due
on Wed., Feb. 22. You can earn up to 5 1S1P pts. The second
is an upper level chart Optional
Assignment due at or before the start of class on Friday, Feb.
24. You can earn 0.5 pts of extra credit and a green card
(if you answer 85% of the questions correctly) on the second assignment.
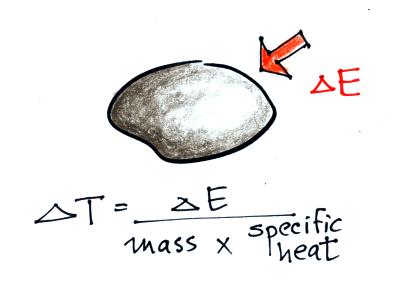
Here's the equation that allows you
to determine how much of a temperature change will occur when energy is
added to or removed from an object.
I brought my propane torch with me to class so that I could heat up the
end of a piece of copper tubing. The figure below
(p. 46 in the ClassNotes) shows you what happens inside an object when
it's temperature changes.
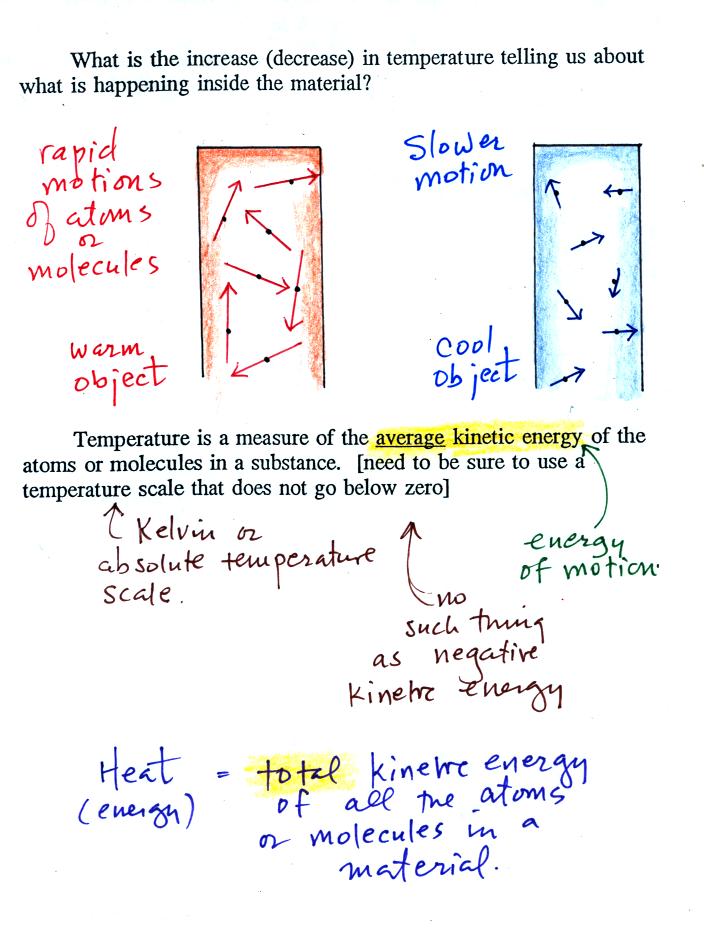
The atoms or molecules inside the
warmer object will be moving more rapidly (they'll be moving freely in
a gas, just "jiggling" around in a solid). Temperature provides a
measure of the average
kinetic energy of the
atoms or
molecules in a material.
You need to be careful what temperature scale you use
when
using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
Speaking of temperature scales
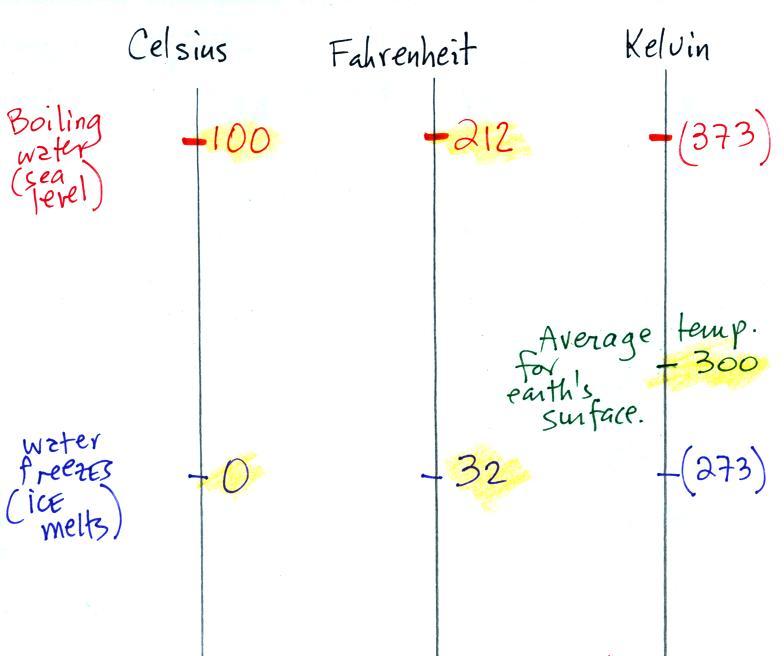
You should remember the
temperatures of the boiling point
and freezing
point of water on at least the Fahrenheit and Celsius scales (and
the Kelvin
scale if you want to). 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth. That's a number you should try to
remember also.
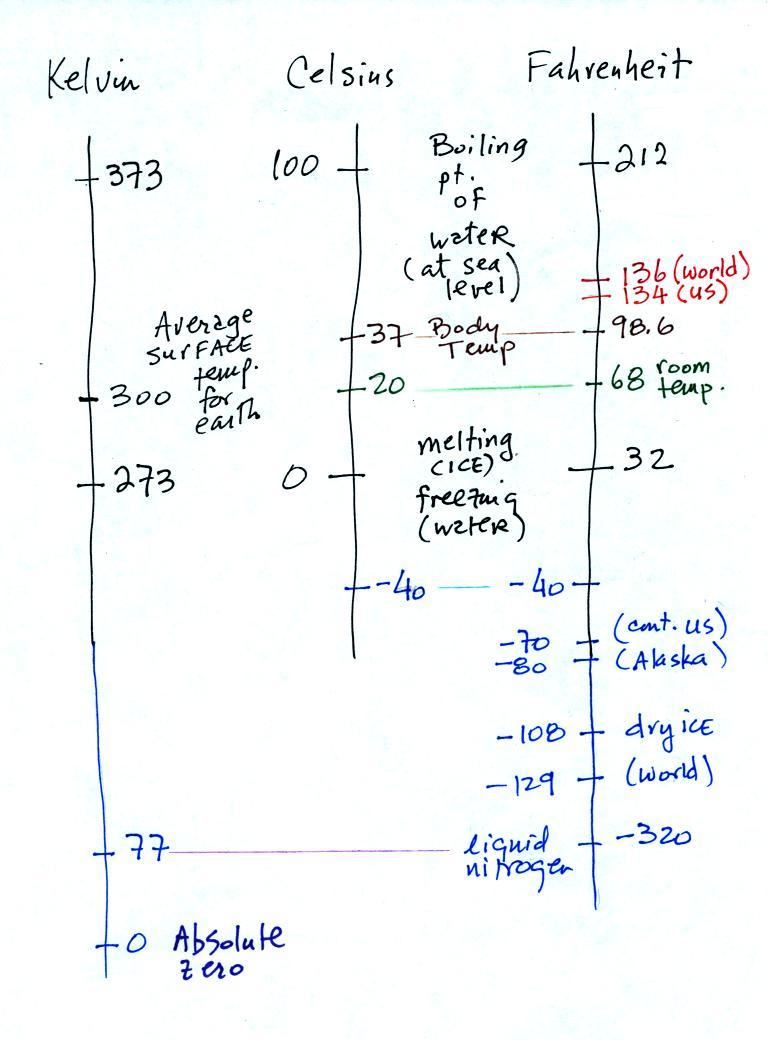
You certainly don't need to try to
remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley (low altitude [below sea level], surrounded by land, and
near 30 degrees latitude). The continental US cold temperature
record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean (remember water moderates climate).
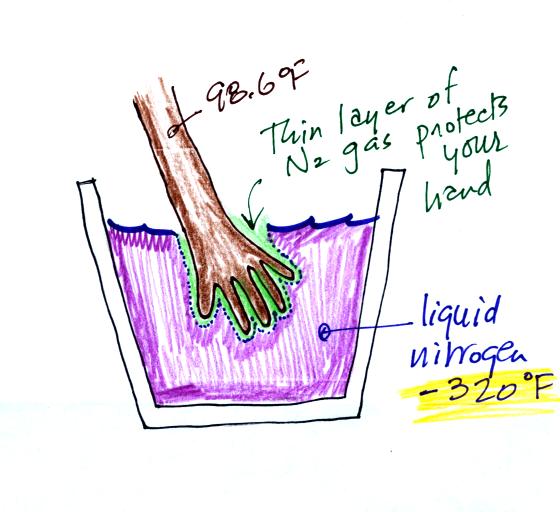
Liquid
nitrogen is cold but it is still quite a bit warmer than absolute
zero. Liquid helium gets within a few degrees of absolute zero,
but it's expensive and there's only a limited amount of helium
available. So I would feel guilty bringing some to class.
This next figure might make clearer the difference between
temperature (average kinetic energy) and heat (total kinetic energy).
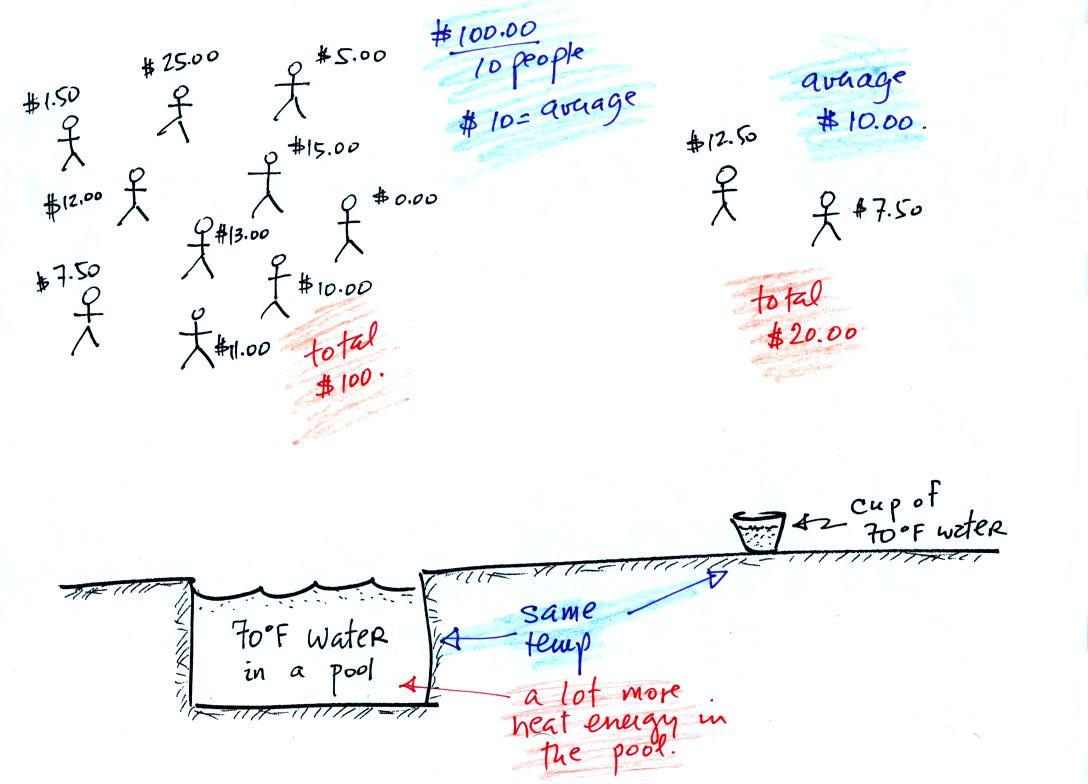
A cup of water and a pool of water
both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people and money (the people
represent atoms or molecules and the money is analogous to kinetic
energy). Both groups have the same same
average
amount
of money per person (that's analogous to temperature). The $100
held by the larger group at the
left is
greater than the $20 total possessed by the smaller group of people on
the right (total amount of money is analogous to heat).
Conduction
is the first of four energy transport processes
that we
will cover (and the least important transport process in the
atmosphere). The figure below illustrates this process. A
hot object is stuck in the middle of some air.
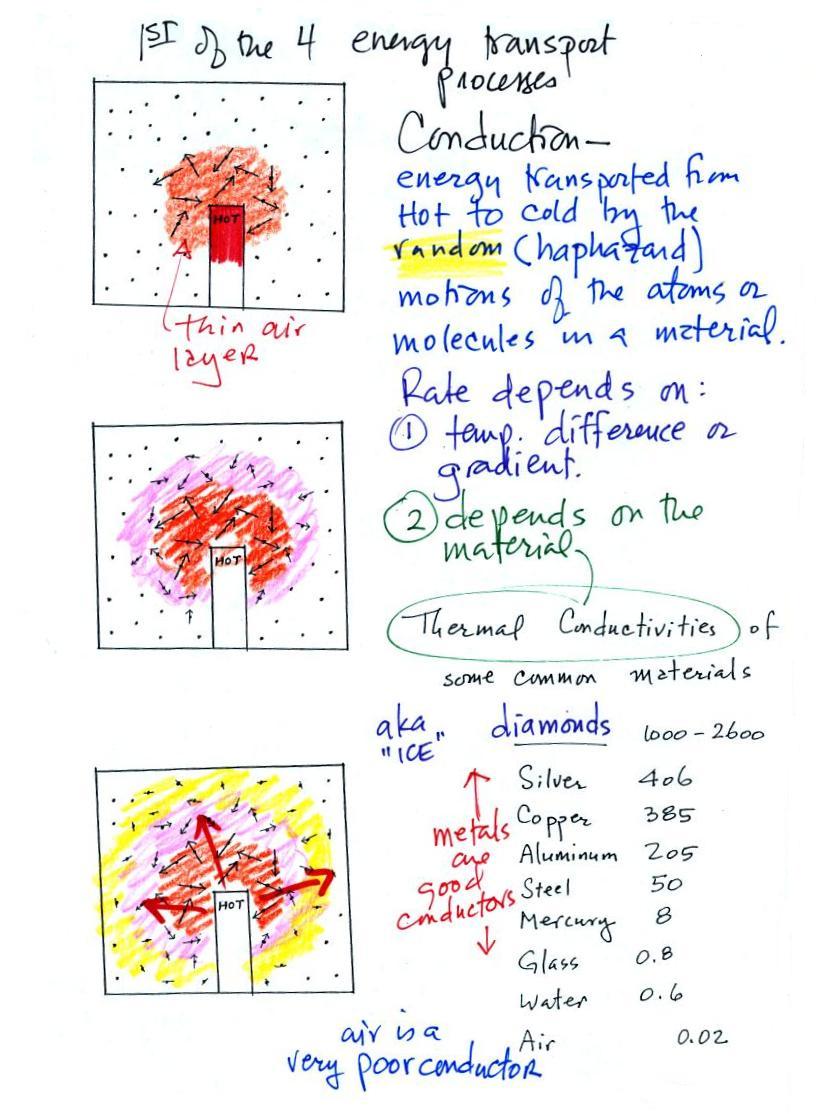
In the top picture some of the
atoms or molecules near the
hot object have collided with the object and picked up energy from the
object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored orange).
In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are pink). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object.
In
the third picture molecules further out (yellow) have now gained
some energy. The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder surrounding
air.
Conduction transports energy from hot to cold. The
rate
of
energy
transport
depends
first
on
the
temperature
gradient
or temperature difference between the hot object and the
cooler surroundings. If the object in the picture
had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding air.
The rate of
energy transport also depends on the material transporting energy (air
in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. I could put my finger alongside the
flame, perhaps half an inch from the propane torch and not feel
anything. Air is
generally regarded as an
insulator.
Water is a little bit better conductor. Metals
are generally very good conductors (cooking pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity (apparently the highest of all known
solids). Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
Transport of energy by conduction is similar to the
transport of a strong smell throughout a classroom by diffusion.
Small eddies of wind in the classroom blow in random directions and
move smells throughout the room. For a demonstration you need
something that has a strong smell but is safe to breathe.
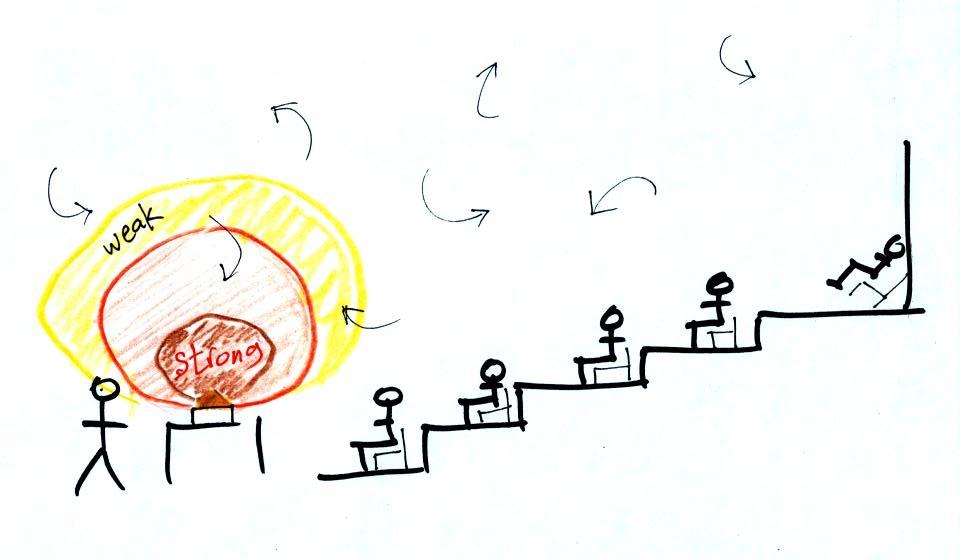
I chose curry powder.
With time I was hoping the smell would
spread
throughout the room. It didn't seem to though. The
room is too big and the ventilation system in ILC 150 is too
good. It
quickly replaces air in the classroom with fresh air from outside (if
mercury were ever spilled I'm guessing the ventilation system won't
allow the vapor to build up the dangerous levels)
Because
air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection (we'll look at convection
shortly). Here are some examples of
insulators that use air:
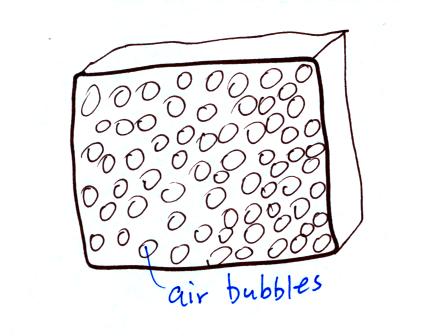
Foam is
filled with lots of small air bubbles, they're what provides the
insulation.
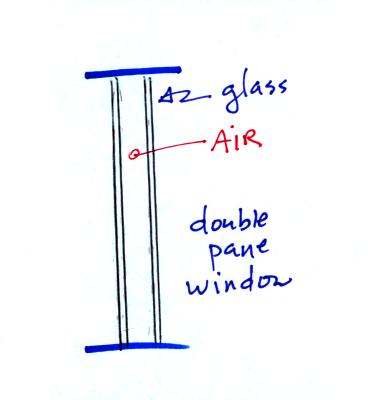
Thin
insulating layer of air in a double
pane window. I don't have double pane
windows in my house. As a matter of fact
I leave a window open so the cats can get in and
out of the house (that's not particularly energy
efficient). And the stray cats have found out about it and come
in to eat my cat's food). Maybe
sprinkling curry powder on the carpet will keep the stray cats out.
Hollow fibers
(Hollofil) filled with air used in
sleeping
bags and
winter coats. Goose feathers
(goosedown) work in a similar way.
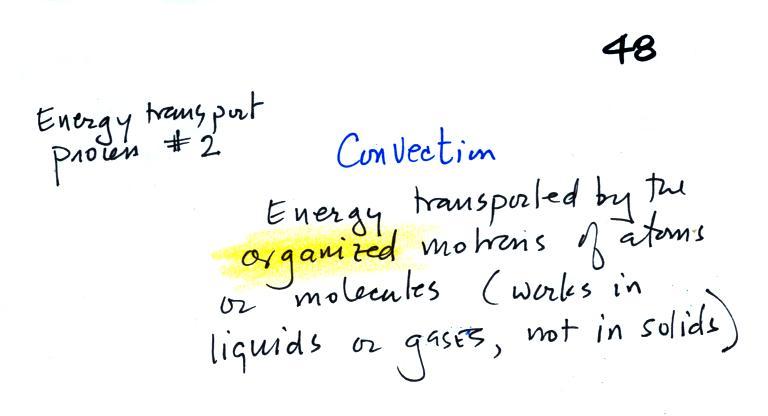
Convection
was the next energy transport process we had a look at.
Rather
than moving about randomly, the atoms or molecules move together as a
group (organized motion). Convection works in liquids and gases
but not
solids (the atoms or molecules in a solid can't move freely).
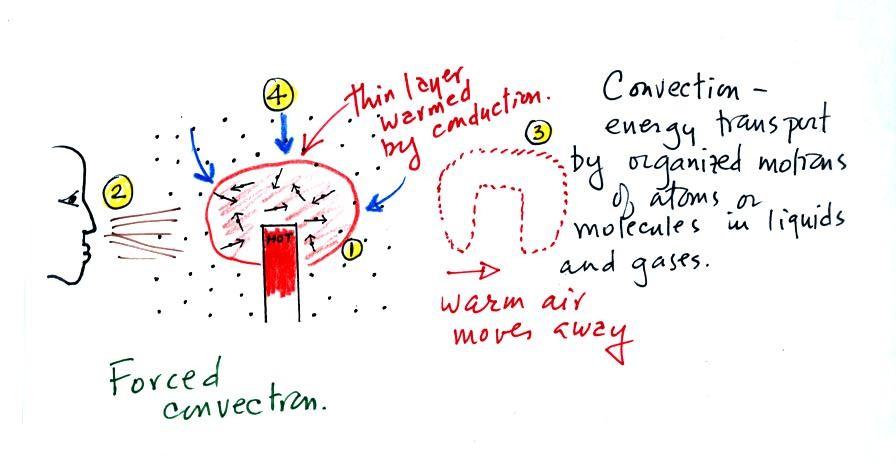
At Point 1 in the picture above a
thin layer of air
surrounding a hot object has
been
heated by conduction. Then at Point 2 a person is blowing the blob of
warm air
off to the right. The warm air molecules are moving away at Point
3 from the
hot object together as a group (that's the organized part of the
motion). At Point 4 cooler air moves in and surrounds the hot
object and the whole process can repeat itself.
This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly. I put a
small fan behind the curry powder to try to help spread the
smell faster and further out into the classroom.
And actually you don't need to force convection, it will often happen
on its own.
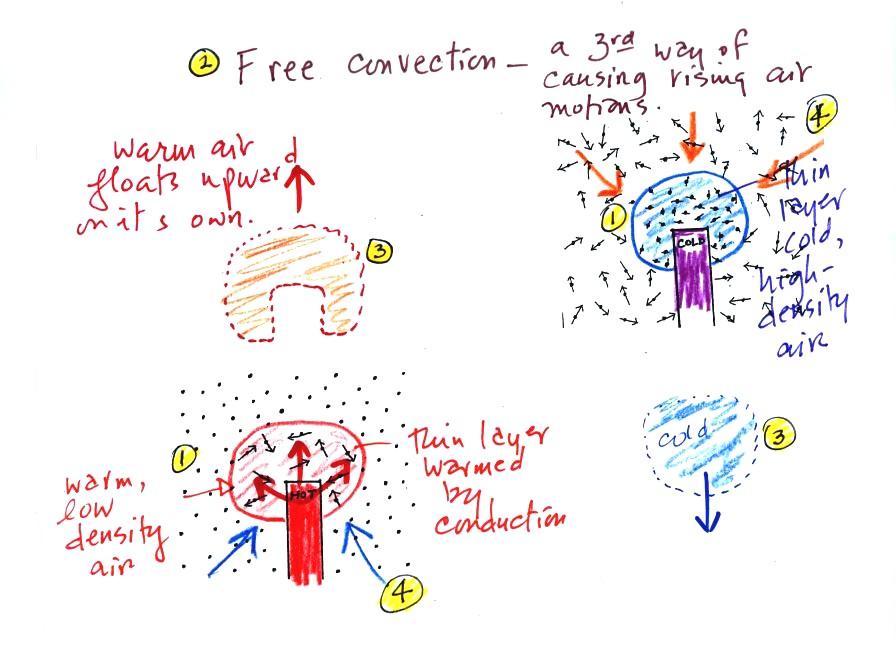
A thin layer of air at Point 1 in
the figure above (lower
left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection. Cooler air moves in to take the place of
the rising air at Point 4 and the cycle repeats itself.
The example at upper right is also
free convection. Room temperature air in contact with a cold
object loses energy and becomes cold high density air. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the room temperature surroundings to the
colder object.
In both examples of free convection, energy is being transported
from
hot toward cold.
I could put my finger alongside the flame from the propane torch
without any problem. There's very little energy transported
sideways through air by conduction. I'm very
careful
if I put my fingers or
hand above the torch. That's something I
forgot to mention. That's because there's a lot of very
hot
air rising from the torch. This is energy transport by
convection. You can see the shimmering rising air when the torch is
held in front of the projector screen.
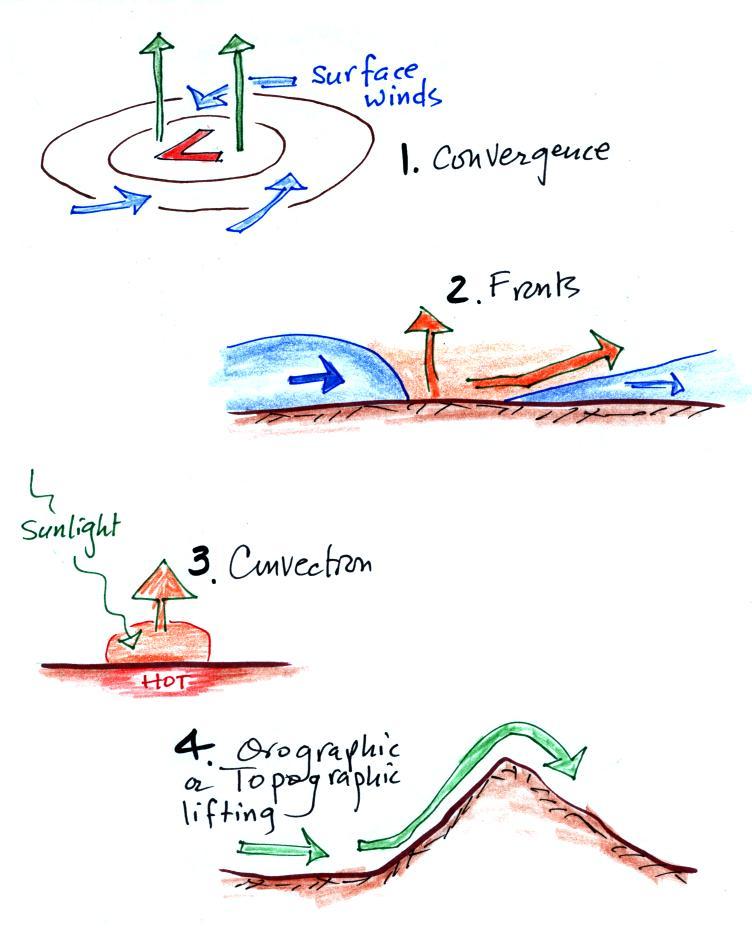
Free convection is a 3rd way of causing rising air motions
(together with convergence into centers of low pressure and
fronts). They're sketched below together with the 4th
process.
Now some fairly practical applications, I think, of what we have
learned about conductive and
convective energy transport. Energy transport really does show up
in a lot more everyday real life situations than you might expect.
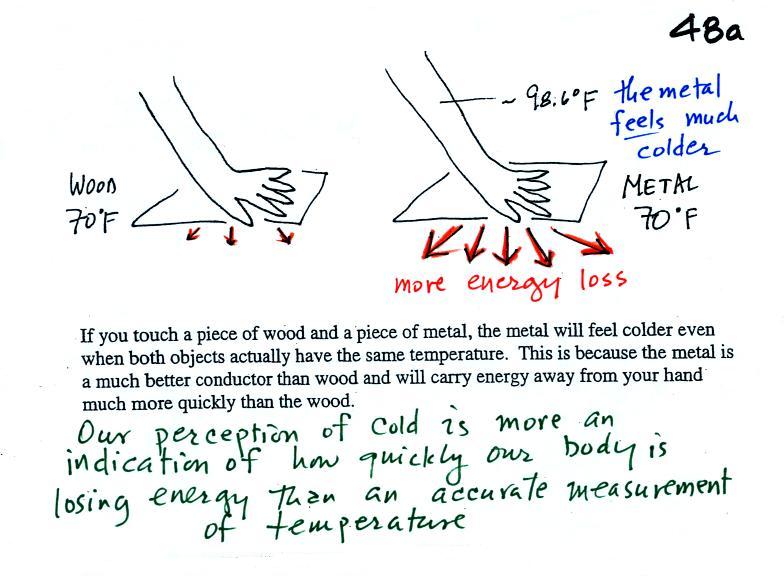
Note first of all there is a temperature difference between
your hand and a room temperature (70 F) object. Energy will flow
from your warm
hand to the colder object. Metals are better conductors than
wood. If you touch a
piece of
70 F metal it will feel much colder than a piece of 70 F wood, even
though they both have the same temperature. A
piece
of 70 F diamond would feel even colder because it is an even better
conductor
than metal. A piece of aluminum and a piece of wood
(oak) were passed around class so that you could check this out for
yourself.
Something that feels cold may not be as
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
Here's a similar situation.
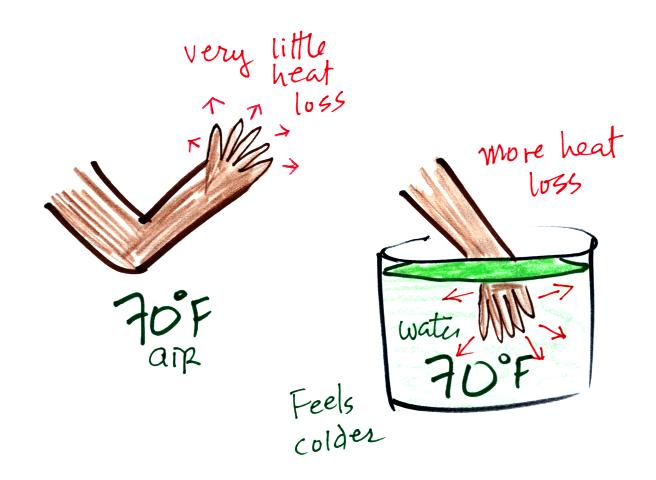
It's pleasant
standing outside on a nice day in 70 F air. But if
you jump into 70 F pool water you
will
feel cold, at least until you "get used" to the water temperature (your
body might reduce blood flow to your extremeties and skin to try to
reduce energy loss).
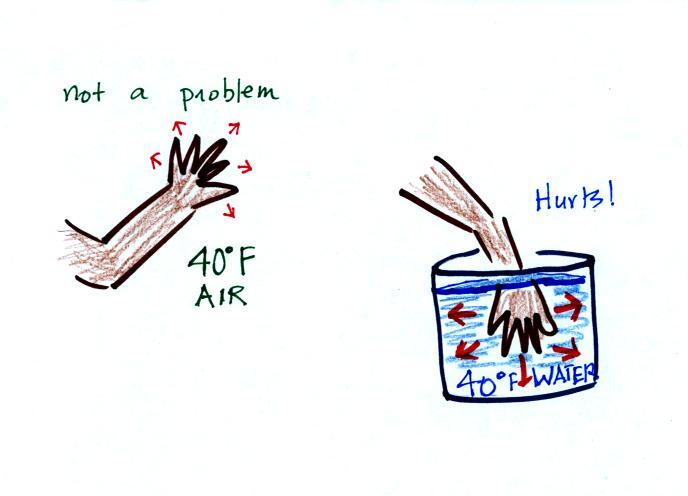
Air is a poor conductor. If you go out in
40 F
weather you will feel colder largely because there is a larger
temperature difference between you and your surroundings (and
temperature difference is one of the factors that affect rate of energy
transport by conduction).
If you stick your hand
into a bucket of 40 F water (I probably shouldn't, but I will suggest
you try this), it will feel very
cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water. Successive application of hot and then cold is
sometimes used to
treat arthritis
joint
pain.