Tuesday, Sep. 17, 2019
We'll be using page 52 and page 53 then page
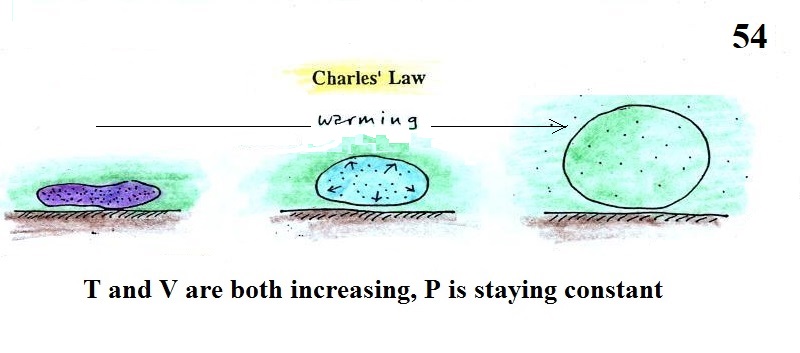
Step #2 Charles Law
A volume of air in the atmosphere is not a rigid container.
Air is free to expand or shrink and will do so in order to keep
the pressures inside and outside the volume in balance.
The figure above is on page
52 in the ClassNotes.
Charles Law refers to situations where P (pressure)
in the ideal gas law stays constant.
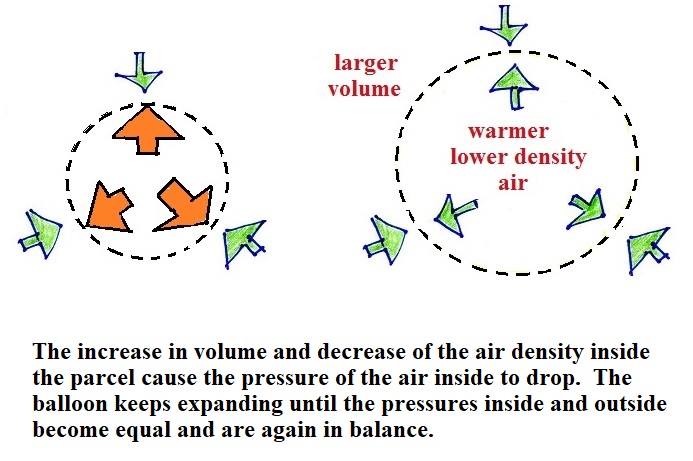
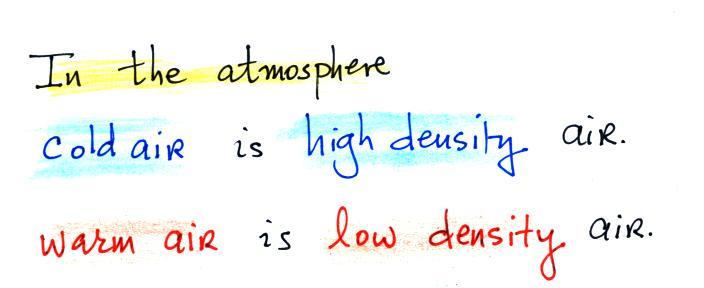
Changing the temperature of a volume of air will cause a change
in density and volume; pressure will stay constant. This is
an important situation because this is how volumes of air in the
atmosphere behave.
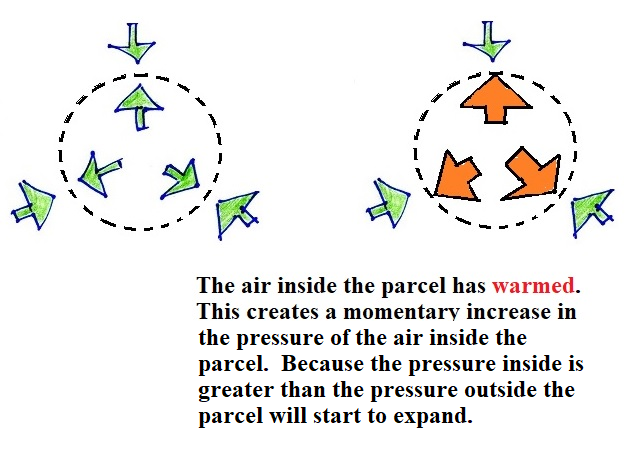
A series of pictures show why and how this happens
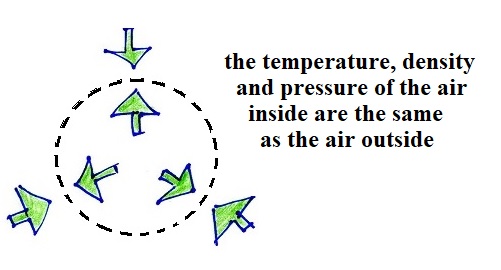
We'll start out with a volume of air. The temperature and
density of the air inside and outside the volume are the
same. So the outward pressure produced by the air inside the
volume is equal to and in balance with the inward pointing
pressure produced by the air surrounding the balloon.
Next we'll warm up the air inside the volume. The air
outside the volume stays the same.
|
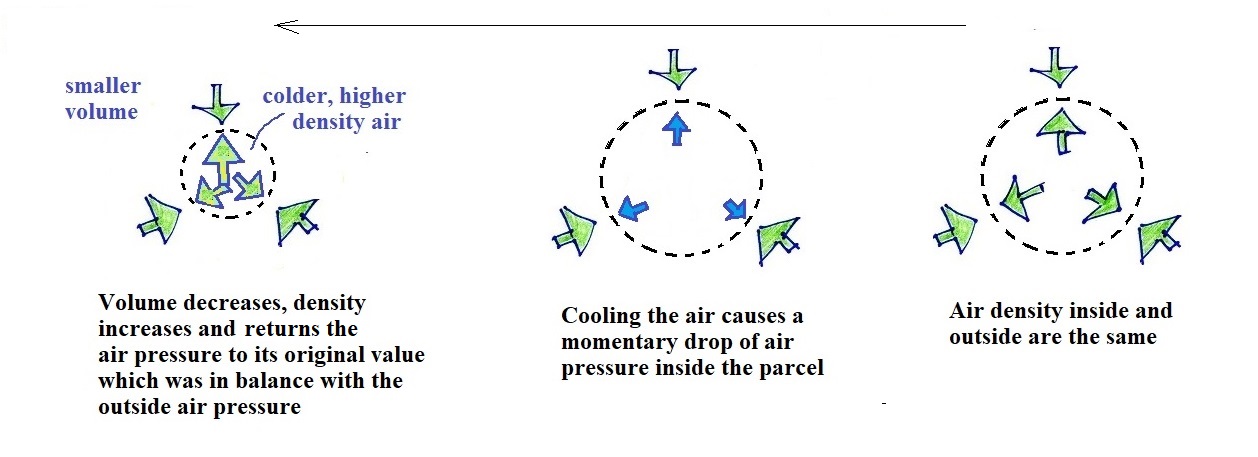
You can go through the same reasoning with a
volume of air that cools.
If you want to skip all the details and just remember one
thing, here's what I'd recommend
Demonstration of Charles Law in action
Parcels of atmospheric air and air in balloons behave the
same way, they both obey Charles' Law. Charles
Law can be demonstrated by dipping a balloon in liquid
nitrogen. You'll find an explanation on the top of p. 54
in the photocopied ClassNotes.
A balloon shrinks down to practically zero volume when
dunked in the liquid nitrogen. When pulled from the
liquid nitrogen the balloon is filled with very cold, very
high density air.
Then the balloon starts to warm up.
The volume and temperature both increasing together
in a way that kept pressure constant (pressure inside the
balloon is staying equal to the air pressure outside the
balloon). Eventually the balloon ends up back at room
temperature (unless it pops while warming up).