Carbon dioxide (greenhouse
gas) and its role in global warming
This is the first of a multi-part series on climate change and
global warming. Here we'll mostly be concerned with carbon
dioxide (CO2), the 5th most abundant gas in the
atmosphere and (together with water vapor) probably
the best known of the greenhouse gases.
It is generally accepted that human activities are causing
the atmospheric concentration of carbon dioxide to slowly
increase (we'll look at some of the experimental evidence in a
moment). The concern is that increasing amounts of CO2
will strengthen the greenhouse effect and cause the earth's
surface to warm.
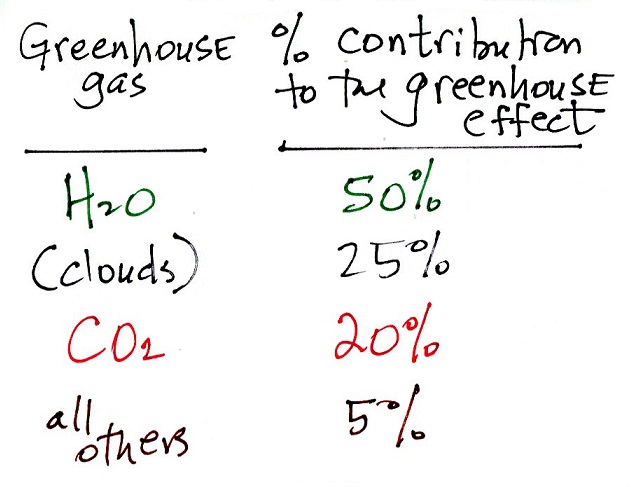
The table above (from this
source in an article
about greenhouse gases in Wikipedia shows the relative
importance of water vapor and carbon dioxide in the greenhouse
effect. Also note the important role of clouds.
Clouds, of course are not made up of gases, rather they are
composed of small drops of liquid water or crystals of ice.
There is still a fair amount of uncertainty about how much
warming will occur, how the warming will vary by region, and about
all the secondary effects that temperature changes may have.
We look at past and predicted future changes in
temperature in the next part of this series.
It is important to remember that the greenhouse effect isn't
all bad, it has a beneficial side. We'll refer to this
as the natural greenhouse effect (i.e. one that has not been
affected or influenced by human activities)
If the earth's atmosphere did not contain any greenhouse gases,
the global annual average surface temperature would be about 0o
F. That's pretty cold and that's the average, there would be
many locations on the earth much colder than that. The presence of
greenhouse gases raises this average temperature to about 60o
F and makes the earth a much more habitable place.
So some warming is a good thing.
Increasing atmospheric greenhouse gas concentrations might
cause some additional warming (and we rely on computer models to
predict or estimate how much warming there will be). This
might not sound like a bad thing. However even a small
change in average temperature might melt polar ice and
cause a rise in sea level which would, at the very
least, pose an environmental threat to coastal areas.
Warming might change weather patterns and bring more precipitation
to some areas and more frequent and more prolonged drought to
other places (like Arizona). Serious tropical diseases (such
as malaria and dengue fever) might spread into areas where they're
not currently found. Plant and animal species might be
forced to migrate in order to find a suitable environment; some
might not be able to adapt quickly enough and could go extinct.
We will save most of the discussion of the effects of global
warming for later in the semester, here we will just
concentrate on carbon dioxide.
Let's first look at some of the experimental data that show
atmospheric carbon dioxide concentration is increasing.
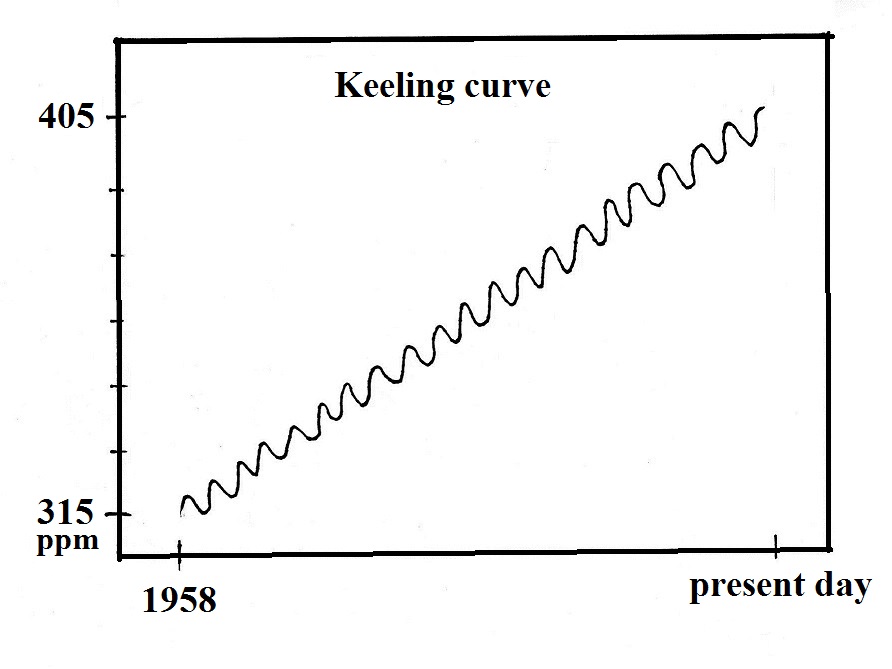
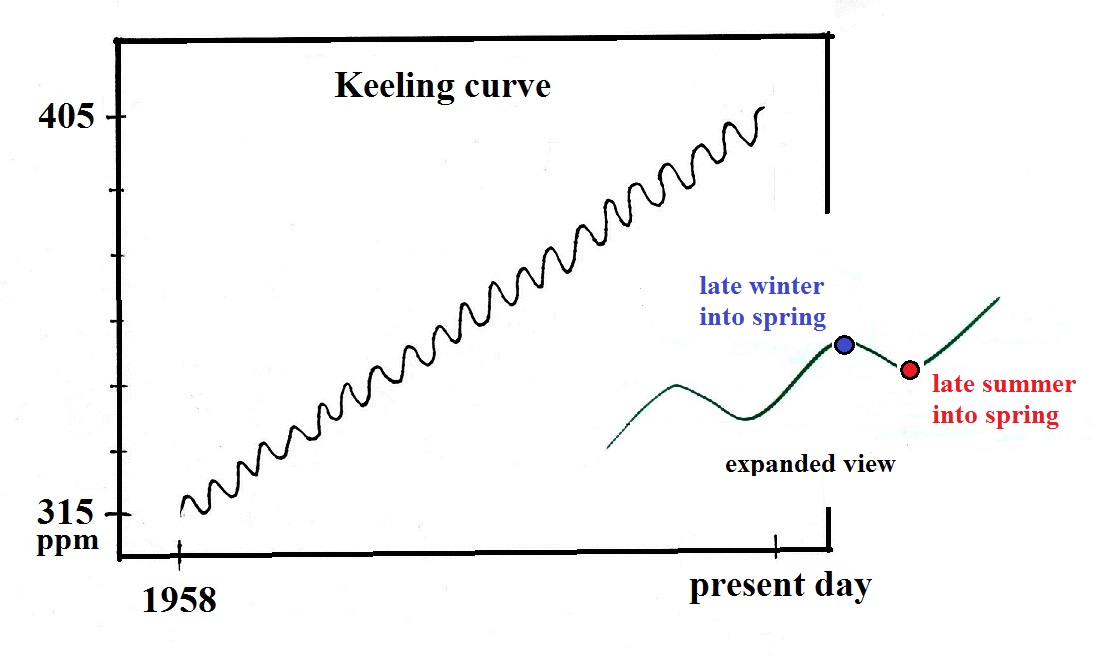
This is a sketch of the "Keeling" curve. I've
purposely left out a lot of key information (labels on the x- and
y-axes, for example). I'm hoping that will cause you to
examine the graph more carefully than you might otherwise, see if
you can figure out or explain what is being depicted.
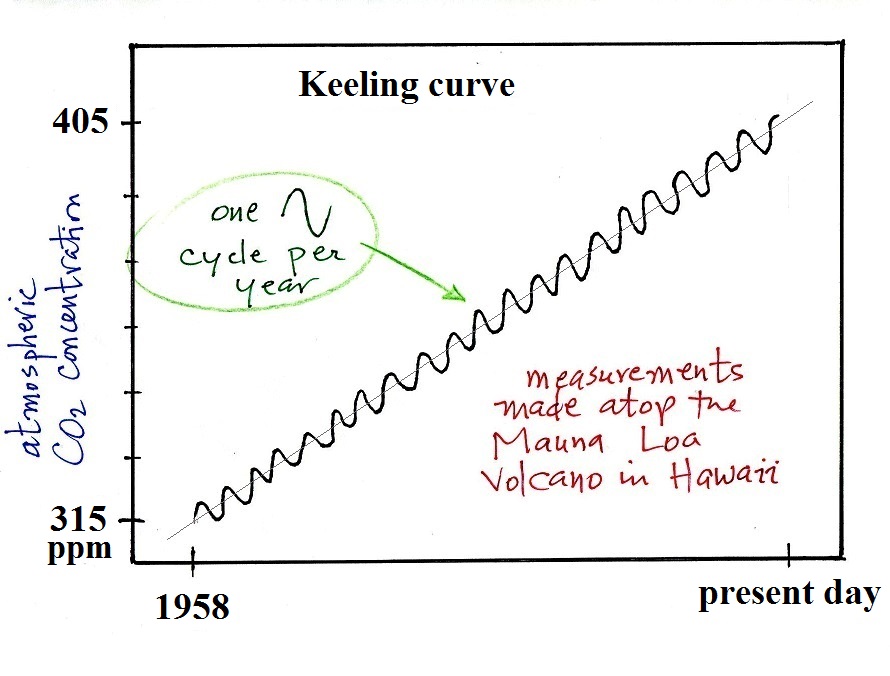
Here's a little more informative version of the Keeling
curve. The graph shows measurements of atmospheric CO2 concentration that were begun
(by a graduate student named Charles Keeling) in 1958 on top of
the Mauna Loa volcano
in Hawaii (the summit is over 13,000 ft above sea level and the
air there is "clean" and not affected by nearby cities and other
sources of pollutants). Carbon dioxide concentration was
about 315 ppm when the measurements began and is now over 400
ppm. The units "ppm" stand for parts per million; 315 ppm
means there are 315 CO2 molecules
mixed in with 1,000,000 (1 million) air molecules (equivalent to
0.0315% concentration).
The small wiggles (one wiggle per year) show that CO2
concentration changes slightly during the course of a year (local
concentrations also changes slightly during the course of a
day). But overall the concentration has been increasing
steadily over the past 50 plus years.
You can find the latest measured atmospheric CO2 concentration at Mauna Loa
Observatory at an interesting,
interactive site maintained by the Scripps Institution of
Oceanography ( links at the bottom of the graph allow you to
display CO2
concentrations on time scales that range from one week to 800,000
years).
 The summit of Mauna Loa is the dark area
The summit of Mauna Loa is the dark area
to the left of center on this image of the "big island"
of Hawaii. (source
of this image)
|
A sideview of the Mauna Loa volcano
( source of
this image)
|
Once scientists saw Keeling's data they began to wonder about
how CO2 concentrations might
have been changing prior to 1958. But how could you now, in
2017 say, go back and measure the amount of CO2 in the atmosphere in the
past? Scientists have found a very clever way of doing just
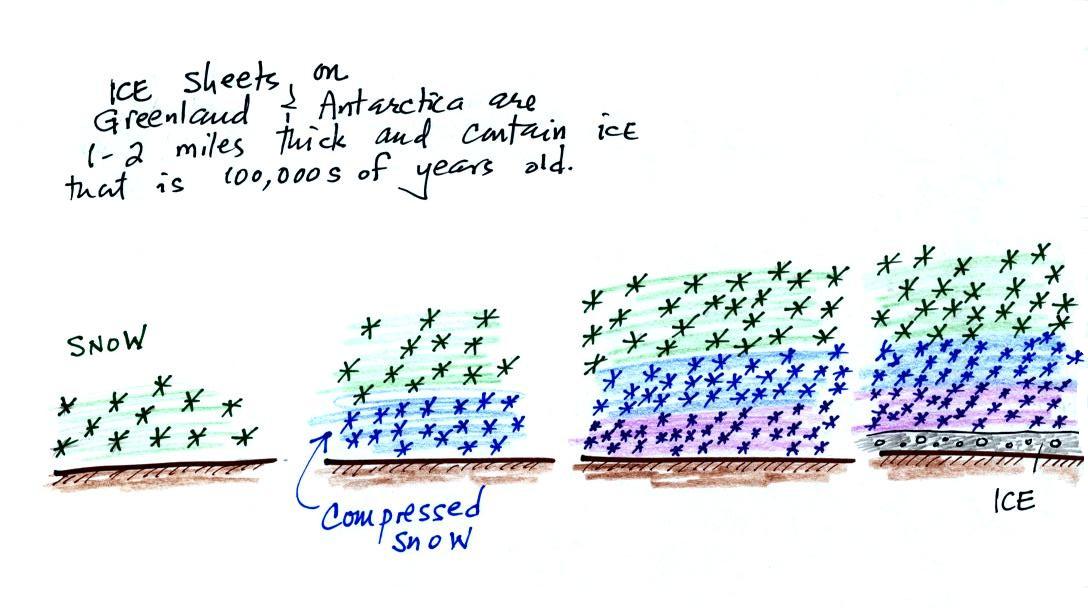
that. It involves coring (drilling) down into ice sheets
that have been building up in Antarctica and Greenland for
hundreds of thousands of years.
As layers of snow are piled on top of each other year after
year, the snow at the bottom is compressed and eventually turns
into a thin layer of solid ice. The ice contains small
bubbles of air trapped in the snow, the bubbles are essentially
sealed samples of the atmosphere at the time the snow originally
fell. Scientists are able to date the ice layers and then
take the air out of these bubbles and measure the carbon dioxide
concentration. This can't be very easy, the layers are very
thin, the bubbles are small and it must be hard to avoid
contamination.
(source
of this image)
Using the ice core measurements scientists
have determined that atmospheric CO2 concentration was fairly constant at about
280 ppm between 0 AD and the mid-1700s when it started to
increase. The start of rising CO2 coincides with the beginning of the
"Industrial Revolution." Combustion of
fossil fuels needed to power factories began to add
significant amounts of CO2
to the atmosphere.
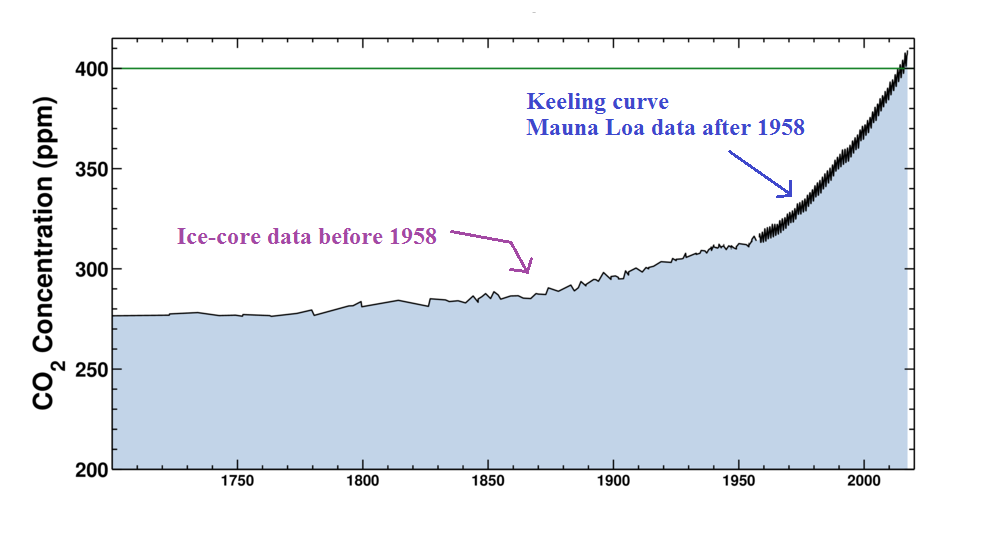
The graph above comes from the Scripps Institute of
Oceanography site.
|
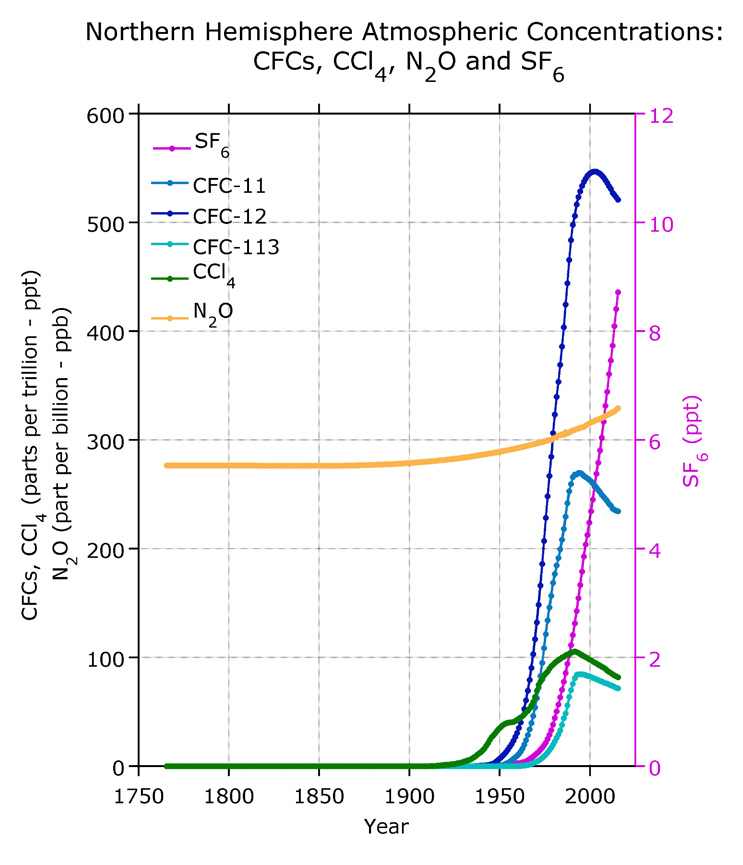
|
| This figure is from Climate
Change
2007, IPCC 4th Assessment Report. |
Source of this figure: Bullister,
J.L. 2015. Atmospheric
Histories (1765-2015) for CFC-11, CFC-12, CFC-113, CCl4,
SF6 and N2O.
Carbon Dioxide Information Analysis Center, Oak Ridge
National Laboratory, US Department of Energy, Oak Ridge,
Tennessee.
|
The figure above at left indicates that atmospheric
concentrations of two other greenhouse gases, methane and nitrous
oxide, have been increasing in much that same way as carbon
dioxide. In addition to being greenhouse gases,
chlorofluorocarbons (CFCs) and carbon tetrachloride (CCl4 ) also react with and destroy
stratospheric ozone (the ozone layer). Because of
international treaties such as the Montreal Protocol, that sought
to phase out substances that are harmful to the ozone layer,
atmospheric concentrations of CFCs and CCl4 have started to decrease.
In order to better understand why
atmospheric carbon dioxide concentration is increasing we need to learn more about how carbon dioxide is
added to and removed from the atmosphere.
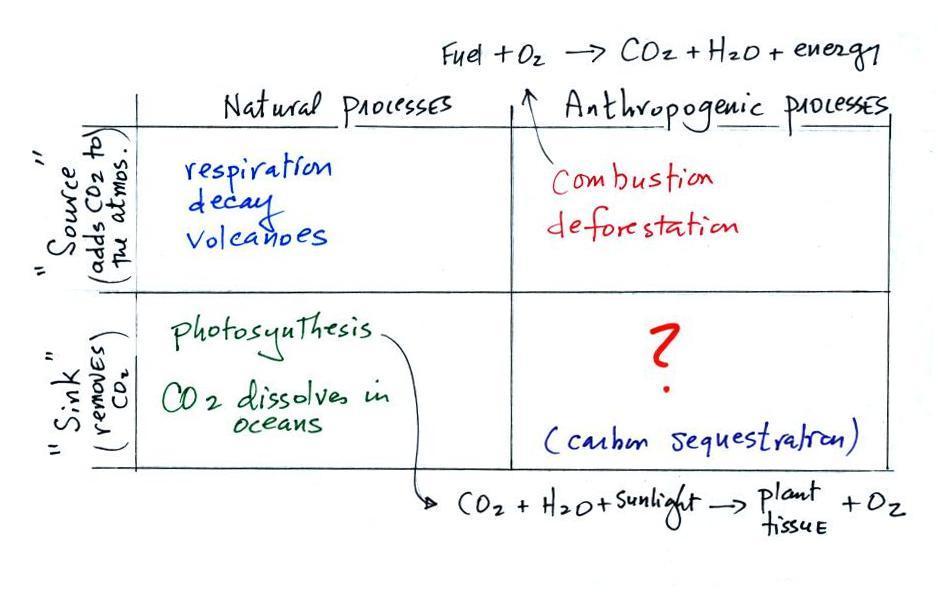
Carbon dioxide is
added to the atmosphere naturally by respiration (animals
breathe in oxygen and exhale carbon dioxide), decay, and
volcanoes.
Combustion of fossil fuels, a human activity also adds CO2
to the atmosphere.
Living vegetation will
remove CO2 from
the air by photosynthesis (the equation shown above).
Killing or cutting down trees, deforestation, will reduce this CO2 removal
process. The
dead tree will also decay and release CO2 to
the air. CO2
also dissolves in the
oceans.
The ? means I'm not not
aware of an anthropogenic process that removes significant
amounts of carbon dioxide from the air. Carbon
sequestration (the capture/removal of CO2 from the air and storage)
is something that is being considered to lessen or prevent
global warming.The amount of emitted depends on the particular
fuel being burned.
Natural gas is a relatively "clean" fuel and releases roughly
half the amount of CO2
per unit of energy generated as coal.
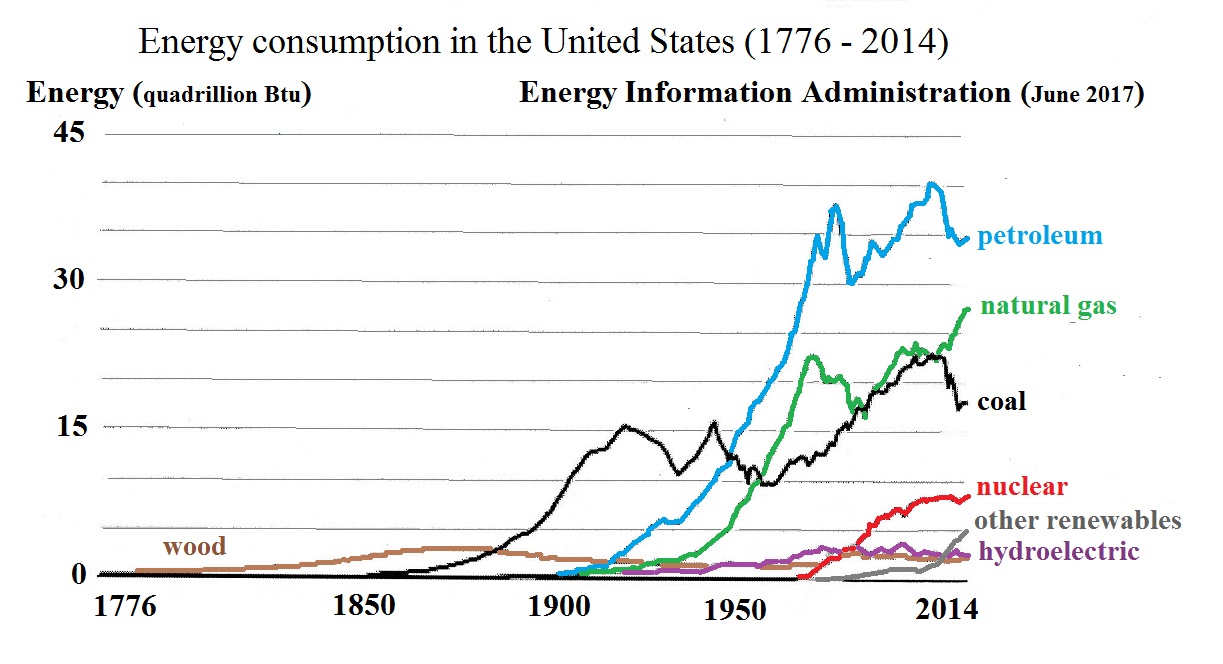
In recent years the
use of coal has started to decrease in the United States and
increasing amounts of energy are being generated by the
combustion of natural gas. Largely because of the shift
from coal to natural gas, emissions of in the United
States have decreased by more than 10% since 2007 (http://www.washingtontimes.com/news/2016/apr/10/stephen-moore-how-fracking-reduces-greenhouse-gase/).
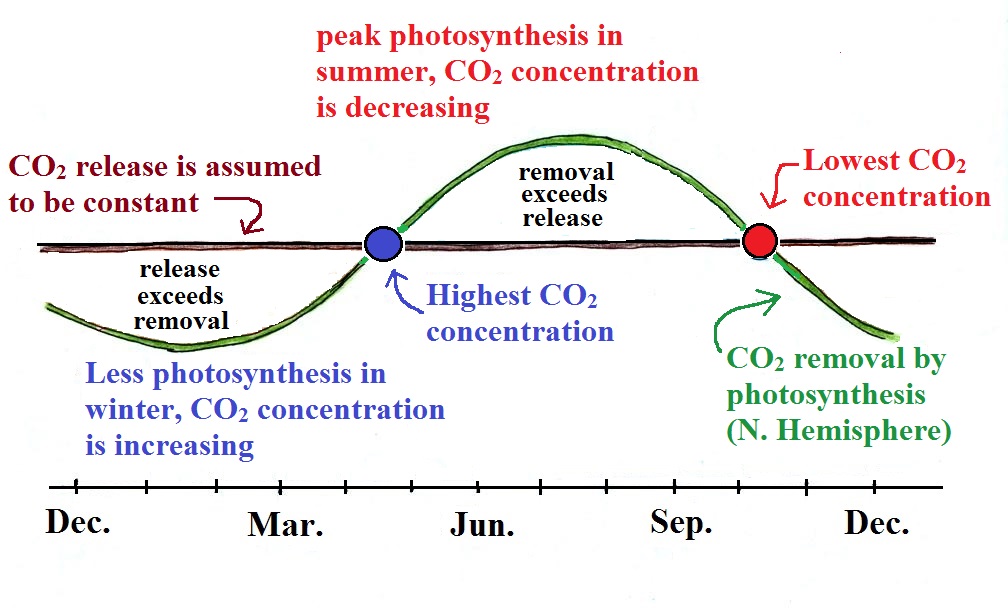
We
are now able to better understand the yearly variation in
atmospheric CO2 concentration (the
"wiggles" on the Keeling Curve) and can figure out when the
highest and lowest CO2 concentrations should occur.
We will assume that the release of CO2 to
the air remains constant throughout the year (the straight brown
line below). The rate that CO2 is removed from the air by
photosynthesis (the green curve) will change.
Photosynthesis is highest in the summer when plants are growing
actively. It is lowest in the winter when many plants are
dead or dormant.
Atmospheric CO2
concentration will decrease as long as the rate of removal
(photosynthesis) is greater than the rate of release (blue shaded
portion above). The minimum occurs at the right end of the
blue shaded portion where the removal and release curves cross.
Once the curves cross and the green photosynthesis curve drops
below the brown curve (rate of release), more CO2 is
being released than removed and the CO2
concentration will start to increase. The highest CO2
concentration occurs once winter is over and the rate of
photosynthesis increases and again becomes equal to the rate of
release. A bank account behaves in the same kind of
way. Assume you are depositing money into the account
at a steady rate but spending varies during the year. The
account balance will rise and fall depending on whether spending
is greater or less than the amount being deposited.
Let's next look at the amounts of carbon
dioxide moving into and out of the atmosphere. A
simplified version of the carbon cycle is shown below (the
next two figures are based on Fig. 6.1 in Climate Change 2013:
The Physical Science Basis available at http://www.ipcc.ch/report/ar5/wg1/).
This figure shows the movement of carbon into and out of the
atmosphere before the beginning of the big industry. This is
what we might have expected to see in the early 1700s.
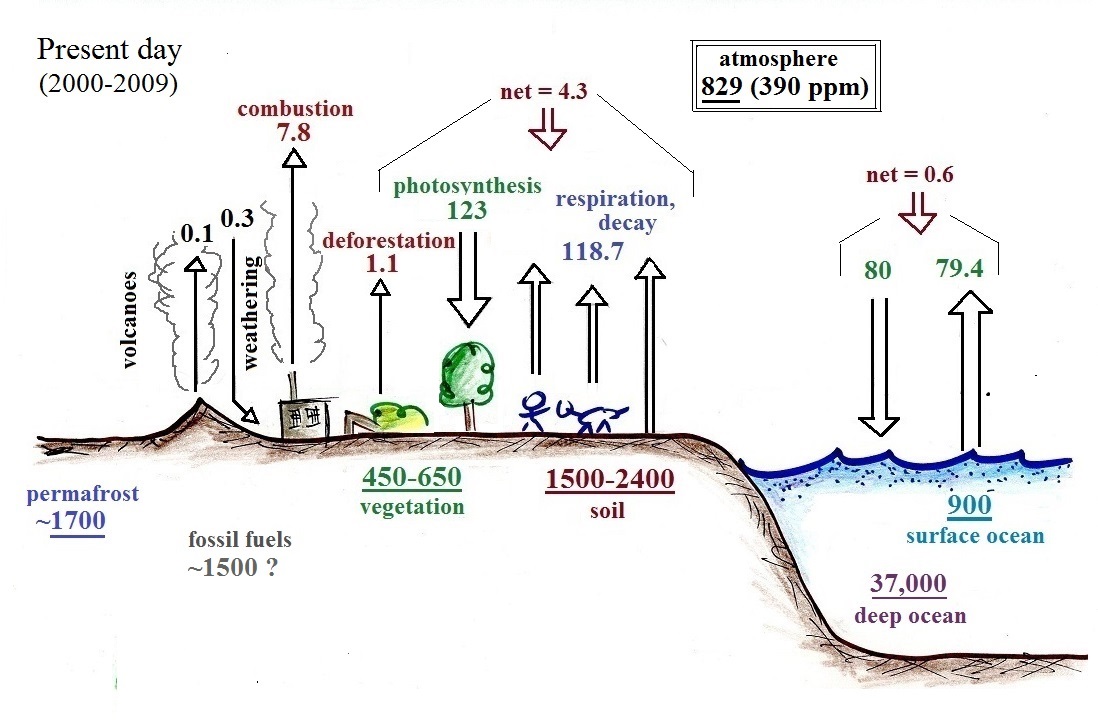
Here are the main points to take from this figure:
1. The arrows show "fluxes," the amounts of
carbon moving into or out of the atmosphere. Over land,
respiration and decay add 107.2 units* of carbon to the atmosphere
every year. Photosynthesis (primarily) removes 108.9 units
every year.
The underlined number shown for the
atmosphere, 589 units, is the amount of carbon stored in the
atmosphere
3. Anthropogenic (man caused)
emissions of carbon into the air are small compared to natural
processes (orange in the figure). About 7.8 units
are added during combustion of fossil fuels (and during the
manufacture of cement) and 1.1 units are added every
year because of deforestation.
The rate at which carbon is added to the atmosphere by man is not
balanced by an equal rate of removal: about half (4.6 of the 8)
units added every year are removed (highlighted in yellow in the
figure).
This small imbalance (8 - 4.6 = 3.6 units of carbon are added to
the atmosphere every year) explains why atmospheric carbon dioxide
concentrations are increasing with time. Note
also that more carbon dioxide is added to the oceans every year
than is removed. Addition of CO2
to the oceans might increase the acidity of the ocean water which
might might make it more difficult for coral and sea shells to
form (shells and coral are made of calcium carbonate CaCO3).
2. Note the natural processes (color coded
blue and green) are pretty much in balance (over land: 120 units
added to the atmosphere and 120 units removed, over the oceans: 90
units added balanced by 90 units of carbon removed from the
atmosphere every year). If these were the only processes present,
the atmospheric concentration (760 units) wouldn't change.
4. In the next 100 years or so, the
7500 units or so of carbon stored in the fossil fuels reservoir
(lower left hand corner of the figure) might be dug up or pumped
out of the ground and burned. That would add 7500 units of
carbon to the air. The big question is how will
the atmospheric concentration change and what effects will that
have on climate? Carbon dioxide can move into and out of the
atmosphere fairly quickly, movement into and out of some of the
other reservoirs is slow. Thus it is difficult to say
precisely how and how quickly the picture will change when it is
perturbed.
*Here they are just in case you are interested:
Reservoirs - Gtons
Fluxes - Gtons/year
A Gton = 1 giga ton = 1012 metric tons. (1
metric ton is 1000 kilograms or about 2200 pounds)
This figure shows the movement of carbon into and out of the
atmosphere.
Here's where we stand at this point:
Atmospheric CO2
concentration was fairly constant between 0 AD and the mid
1700s but has been has been increasing since the mid 1700s
largely due to human caused activities. The obvious
question is what has the temperature of the earth been doing
during this period? In particular has there been any warming
associated with the increases in greenhouse gases that have
occurred since the mid 1700s? The answer to that
question will be the subject of the next part in this series.
CO2 poisoning. Is CO2
a pollutant?
Before we leave this topic a little more information about
carbon dioxide. Up to this point we've been interested
in CO2 because of its role in the atmospheric
greenhouse effect. I generally don't consider CO2
to be an air pollutant because the atmospheric concentration
is small and its not a toxic gas. Under certain
circumstances however CO2
can build to unhealthy even deadly levels (and it kills mainly
because your body is starved of oxygen).
Here is a brief summary of the physiological conditions that
can occur because of exposure to elevated CO2
levels (adapted from http://en.wikipedia.org/wiki/Carbon_dioxide).
For comparison, keep in mind the atmospheric concentration of
carbon dioxide is about 400 ppm (0.04%).
concentration
|
physiological symptoms
|
1% (10,000 ppm)
|
some people start to experience
drowsiness.
|
2%
|
mildly narcotic, increases blood
pressure and pulse rate, and decreases hearing
|
5%
|
shortness of breath, dizziness,
confusion, anxiety, headache
|
8%
|
dimmed sight, sweating, muscular
tremors, loss of consciousness after 5 to 10 minutes
exposure
|
The Occupational Health and Safety Administration (OSHA) has set a
permissible 8 hour working day exposure limit of 5000 ppm (0.5
%). CO2 concentration is sometimes
measured/monitored in work areas to insure that ventilation
systems are adequate and working properly.
Submarines (and spacecraft) are one place where carbon dioxide
levels could potentially build to dangerous levels and scrubbers
are used to remove CO2 from the air and keep CO2
levels within acceptable levels (generally less than 8000 ppm) You
may remember that one of the problems faced by the Apollo 13 crew
was jury rigging a scrubber to keep the carbon dioxide inside
their spacecraft within acceptable levels.
Carbon dioxide poisoning ("blackdamp") is one of several hazards
that miners face when working underground. And this is, as
best I can tell, the reason miners used to carry a caged canary
into the mine with them. Birds are more sensitive to carbon
dioxide than humans and the canary would stop singing and fall off
its perch if CO2 levels were too high. Carbon
dioxide is also involved in a rare type of natural disaster called
a lake overturn or limnic
eruption. What happens here is that cold water at the
bottom of a lake containing dissolved CO2 is suddenly
forced to the lake surface and releases its CO2 (like
the bubbles coming from a carbonated beverage when opened).
Carbon dioxide is heavier than air and is odorless. People and
animals near the lake maybe unaware of the release and buildup of
CO2 and can suffocate. According the Wikipedia
article cited above, events like this have apparently only been
observed twice: in Cameroon at Lake Monoun in 1984 (causing the
death of 37 people living nearby) and in 1986 at nearby Lake Nyos
where around 1200 people were killed.
Finally brief mention of a recent archaeological discovery:
Pluto's Gate to Hell (the god of the underworld was named Pluto by
the Romans and Hades by the Greeks). Pluto's Gate was
considered in classical times to be the entrance to the underworld
(one of many perhaps) and was discovered in 2013 by Italian
archaeologists at the ancient city of Hierapolis in southwestern
Turkey.

|

|
The site as it appears
now (source
of this photograph)
|
The site as it might have
appeared in ancient times. This photograph,
credited to Francesco D'Andria, the Italian archaeologist
that announced the discovery in March, 2013, is found in a news
report from the National Geographic Society.
|
The "gate" was built on top of a cavern and, in ancient times, a
mist of deadly vapors could be seen coming from the cave.
Here's a quote from the Slate
article where I first read about the discovery: "Two
millennia ago, visitors to Pluto's Gate could buy small birds or
other animals (the sale of which supported the temple) and test
out the toxic air that blew out of the mysterious cavern.
Only the priests, high and hallucinating on the fumes, could stand
on the steps by the opening to hell. They would sometimes
lead sacrificial bulls inside, later pulling out their dead bodies
in front of an awed crowd.
As the Greek geographer, philosopher, and prolific traveler
Strabo, who lived from 64/63 B.C. to 24 A.D., so enticingly
described it: 'This space is full of a vapor so misty and dense
that one can scarcely see the ground. Any animal that passes
inside meets instant death. I threw in sparrows and they
immediately breathed their last and fell.' Can you guess what such
a deadly gas might be escaping from Pluto's Gate? Carbon
dioxide.