Friday, Mar. 30, 2007
The Experiment #4 reports are due next
Monday. If you have returned your materials yet and picked up the
supplementary information sheet, Monday morning will be your last
opportunity. You will need to come to my office (PAS 588) leave
your materials in the box just inside the door to the left, and help
yourself to a copy of the supplementary information sheet.
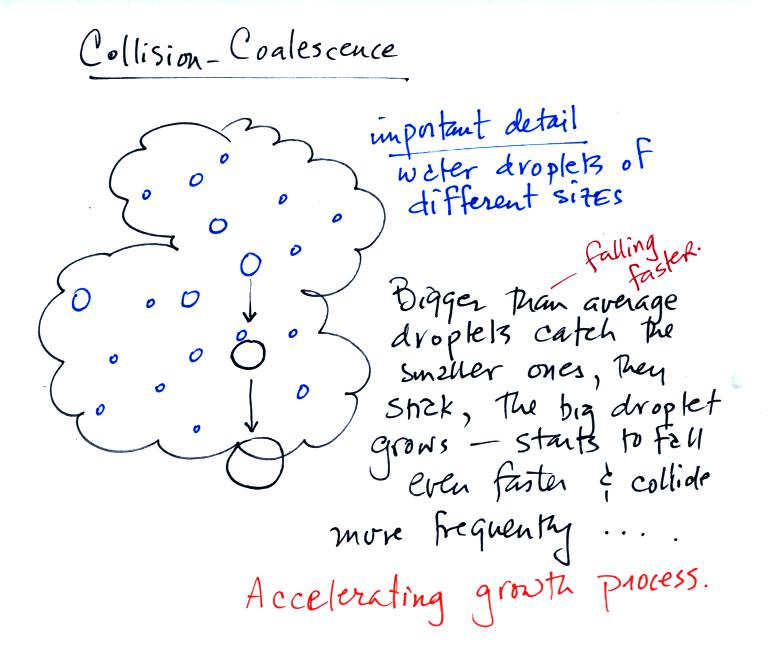
The collision coalescence process works best in a cloud
filled with cloud droplets of different sizes. As we saw in a
short video the larger droplets fall
faster than the small droplets. The large droplets overtake and
collide with the smaller ones. The droplets then stick together and
form any even larger droplet that will fall faster than before and
sweep out a larger volume. In this accelerating growth process an
above-average size droplet can
quickly turn into a raindrop.
The figure below shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns clouds are thinner
and have weaker updrafts than Cb clouds. The largest raindrops
fall from Cb clouds because the droplets spend more time in the cloud
growing.
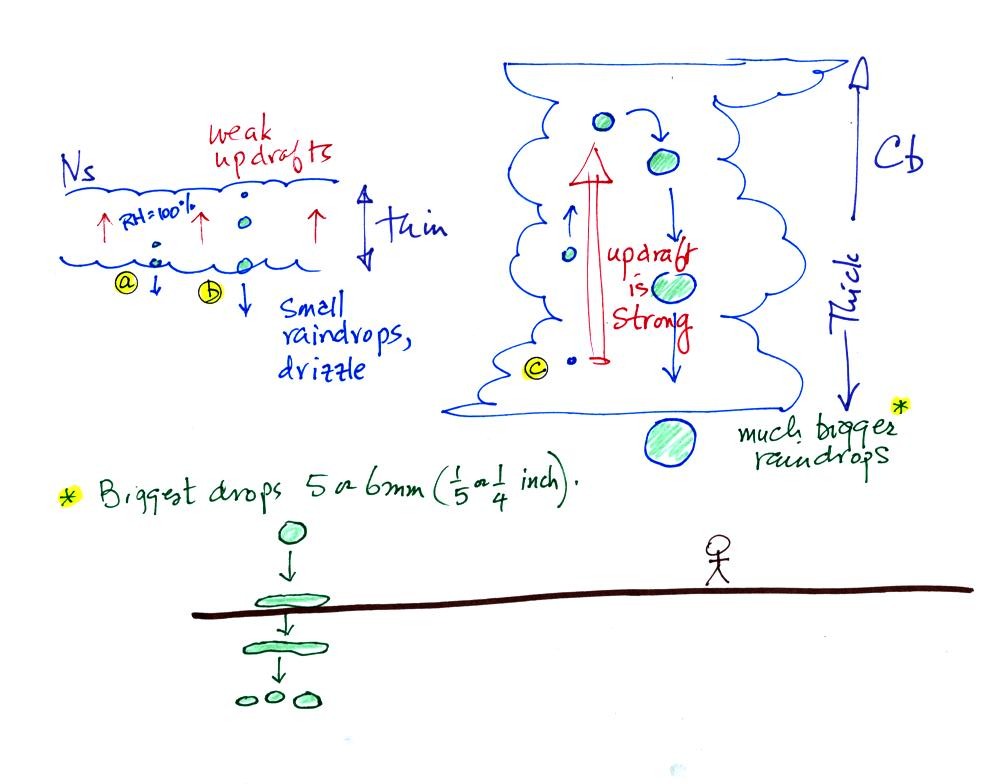
Raindrops grow up to about 1/4 inch in diameter. When drops get
larger than that, wind resistance flattens out the drop as it falls
toward the ground. The drop becomes unstable and breaks apart
into several smaller droplets. Solid precipitation particles that
are made of ice can get much larger than 1/4 inch in diamter.
Before
learning about the second precipitation producing process, the ice
crystal process, we need to look at the structure of cold clouds.
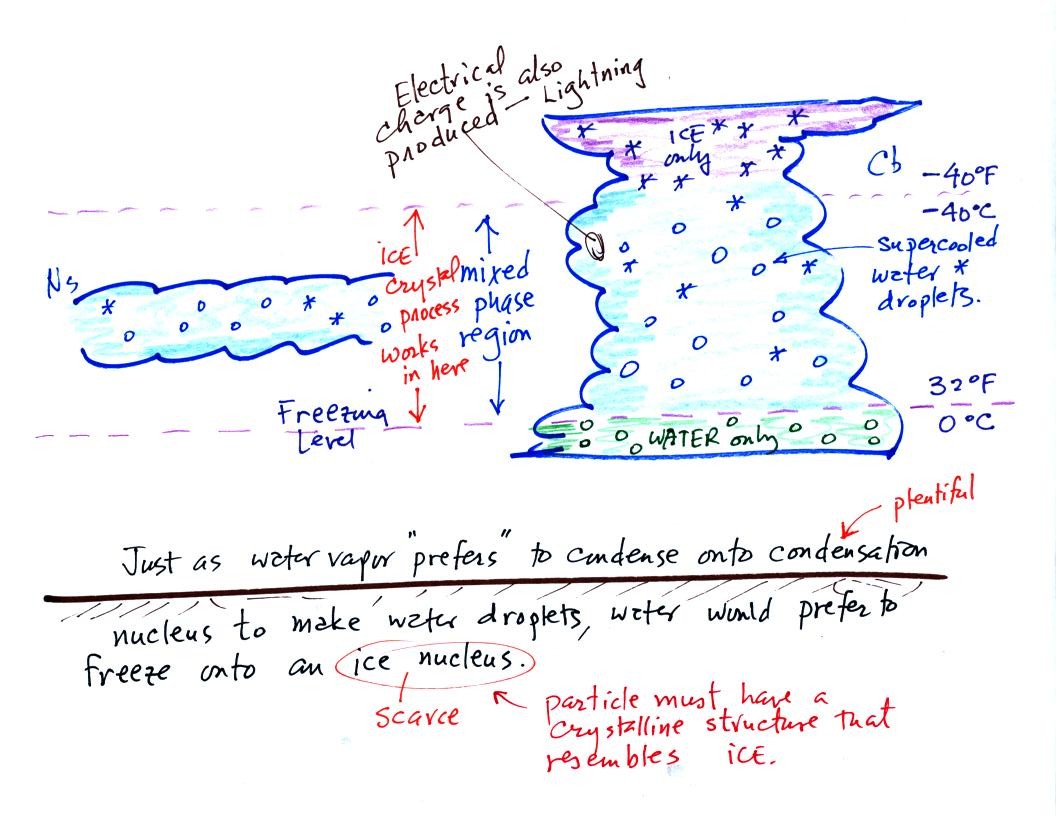
The top of the thunderstorm is so cold (colder than -40 F) that there
are just ice crystals
there. The bottom is warm enough (warmer than freezing) to just
contain water
droplets. The interesting part of the thunderstorm and the
nimbostratus cloud is the part that contains both supercooled water
droplets (water that has
been cooled to below freezing but hasn't frozen) and ice
crystals.
This is called the mixed phase
region. This is where the ice crystal process will be able
to produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
The supercooled water droplets aren't able to freeze even though
they
have been cooled below freezing. This is because it is much
easier for small droplets of water to freeze onto an ice crystal
nucleus (just like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small droplet
of pure water). Not just any material will work as an ice nucleus
however. The material must have
a crystalline structure that is like that of ice.
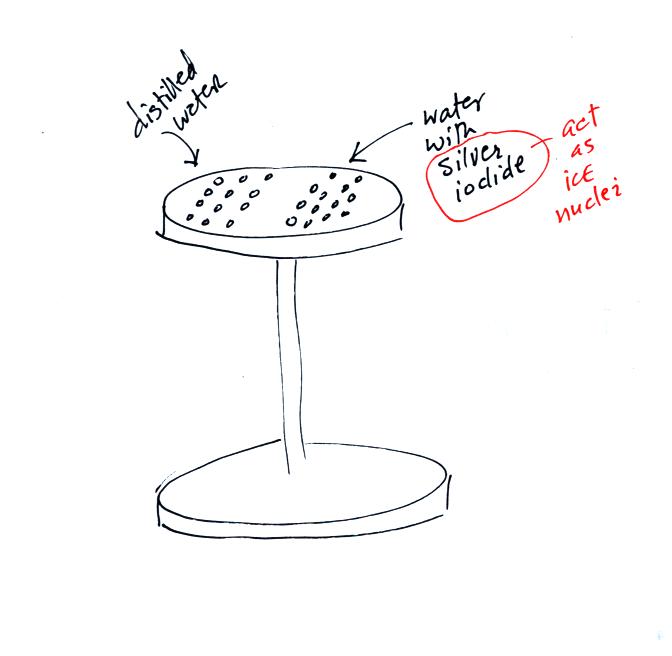
Back before there was NATS 101 there was a course called ATMO 171
(also an Introduction to Weather and Climate class) and a one hour lab
course ATMO 171L. The figure above shows an experiment the ATMO
171L students used to do. The students would put a bunch of
distilled water drops on one side of an aluminum stand. They
would put an equal number of water drops containing silver iodide on
the other side of the stand. Silver iodide is one of the unusual
materials that acts as an ice nucleus.
The stand was put into a styrofoam ice chest and some liquid nitrogen
was poured in to cool the stand. The students could monitor the
temperature of the top platform using a thermometer stuck into a hole
drilled in the side (not shown in the figure above).
We watched a short video showing the freezing of the drops. The
water drops containing silver iodide froze before the distilled water
drops. The silver iodide drops still had to be cooled to be 0 C
before freezing (average freezing temperature was around -5 to -10
C). The average freezing temperature of the distilled water drops
was between -10 and -20 C.
Now we'll
see how the ice crystal process works.

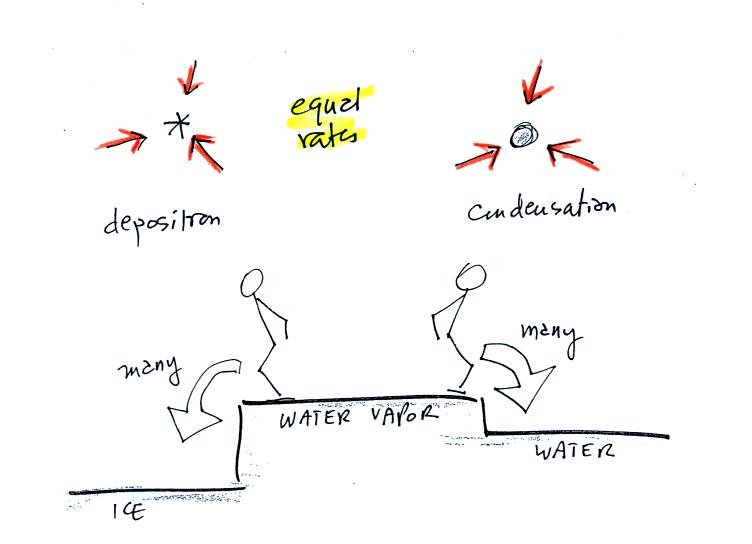
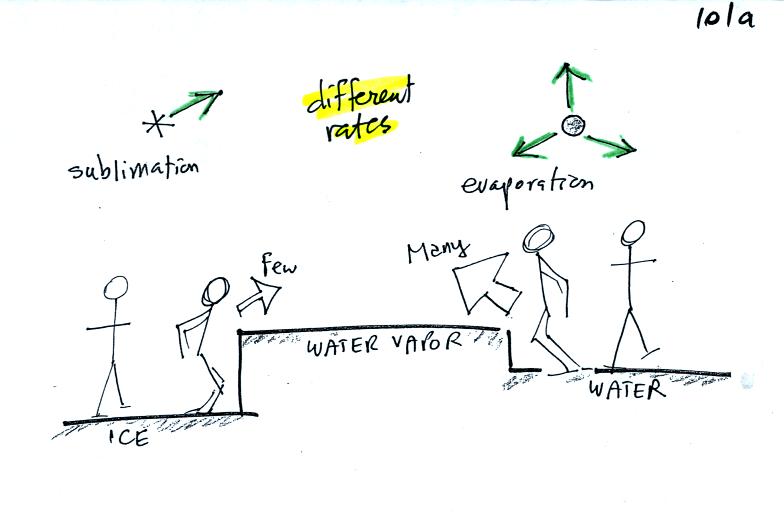
The first figure above (see p.101 in the photocopied Class Notes)
shows a water droplet in equilibrium with its surroundings..The droplet
is evaporating (the 3 green arrows in the figure). The rate of
evaporation will depend on the temperature of the water droplet.
The droplet is surrounded by air that is saturated with water vapor
(the droplet is inside a cloud where the relative humidity is
100%). This means there is enough water vapor to be able to
supply 3 arrows of condensation.
The next figure shows what is required for an ice crystal (at the same
temperature) to be in equilibrium with its surroundings. First
the ice crystal won't evaporate as rapidly as the water droplet (only
one arrow is shown). Going from ice to water vapor is a bigger
jump than going from water to water vapor. There won't be as many
ice molecules with enough energy to make that jump. A sort of
analogous situation is shown in the figure below (many people could
jump up a 1 foot step, fewer people would be able to jump up a 3 foot
step).
To be in equilibrium only one arrow of condensation is needed.
There doesn't need to be as much water vapor in the air surrounding the
ice crystal to supply this lower rate of condensation.
Now what happens in the mixed phase region of a cold cloud is that
ice crystals find themselves in the very moist surroundings needed for
water droplet equilibrium. This is shown below.
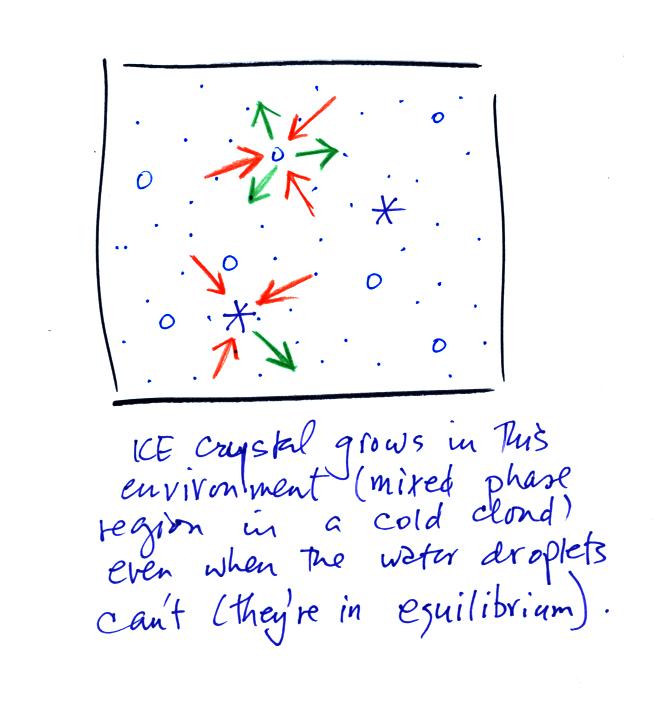
The water droplet is in equilibrium (3 arrows of evaporation and 3
arrows of condensation) with the surroundings. The ice crystal is
evaporating more slowly than the water droplet. Because the ice
crystal is in the same surroundings as the water droplet water vapor
will be condensing onto the ice crystal at the same rate as onto the
water droplet. The ice crystal isn't in equilibrium, condensation
exceeds evaporation and the ice crystal will grow.
The equal rates of condensation are shown in the figure below using the
earlier analogy.