Here is some information on sulfur dioxide. The figures
below are found on pps 11&12 in the photocopied class notes.
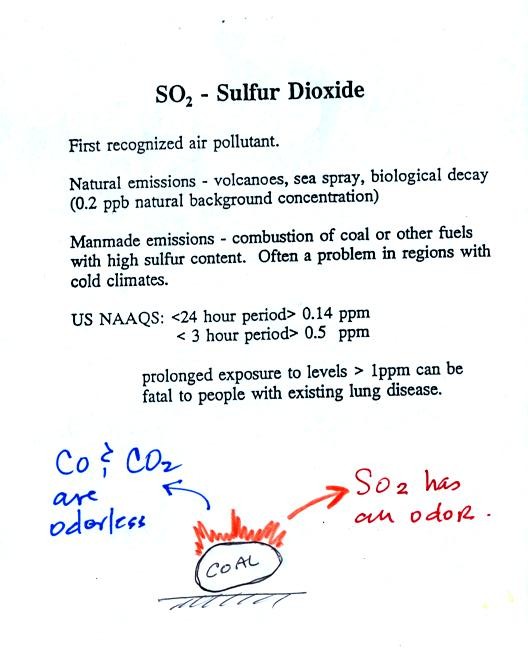
Sulfur dioxide is produced by the combustion of sulfur
containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless.
Volcanoes are a natural source of sulfur dioxide.

The Great London smog is still the deadliest air pollution
event in
history. Because the atmosphere was stable, SO2
emitted into air
at ground level couldn't mix with cleaner air above. The SO2
concentration was able to build to dangerous levels. You'll find
the Donora, PA, disaster described in more detail on p. 335 in the text.
London type smog which contains sulfur dioxide and is most common
during the winter is very different from photochemical or Los Angeles
type smog. Los Angeles type smog contains ozone and is most
common in the summer.
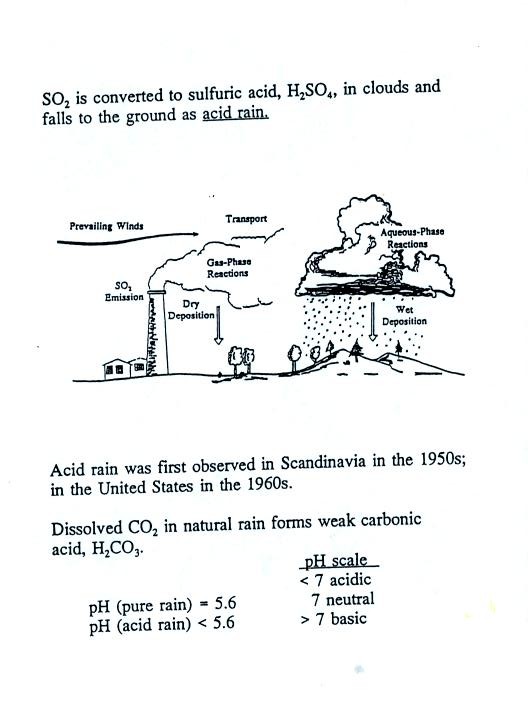
Acid rain often falls hundreds or
thousands of miles away from the
source of the SO2. Coal fired factories and electric
power plants
in the Ohio River Valley could produce acid rain in New England and
Canada. Acid rain in Scandinavia could be the result of SO2
emissions in England and Belgium. Oxides of nitrogen (NO, NO2,
and N2O) also react in clouds to form acid rain (nitric
acid).

Among other things, acid rain has slowly been destroying stone
buildings, statues and monuments. The acid rain changes the
relatively durable limestone or marble to gypsum which is more readily
dissolved and washed away by rain.



