The energy state of a gas depends on its
Later we will see that these three measurable properties of gases are related to each other by the ideal gas law.
For now we will first describe the macroscopic properties of gases in terms of what is going on at the molecular or microscopic level using the kinetic model. Link to page describing kinetic model.
As we describe how each of these three properties of gases can be understood using the kinetic model, we will examine how each changes in the vertical, i.e., as one moves up and down in the real atmosphere.
Temperature is determined by the average speed of the molecules making up a substance. The higher the temperature, the faster they move. For gases, this is the random motion of the individual molecules that make up the gas. Random motion is disordered, i.e., individual molecules are equally likely to be moving in any direction. At temperatures common in Earth's atmosphere, the average speed of each molecule is approximately 1000 mi/hr. This is different from what we call "wind" which is ordered movement of air at the macro scale (basically the ordered movement of a fluid in a given direction). For example, when the windspeed is 10 mi/hr, it means that "blobs" of air are moving at 10 mi/hr, but individual molecules are still moving at an average speed of about 1000 mi/hr.
Using the concept of energy, the higher the temperature, the more energy that is possessed by the gas. It should make sense, then, that the higher the temperature, the higher the energy, and the faster the speed at which the molecules are moving.
We sense temperature by touch. Thermorecptor nerve cells in our body are sensitive to the average speed at which air molecules are moving. Similarly, when air molecules strike a thermometer, energy is transferred between the thermometer and the air. The reading on the thermometer is calibrated to read the average thermal motion of all of the air molecules that collide with it.
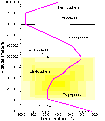
Often, the Earth's atmosphere is divided into several different layers that can be defined according to air temperature.
 |
 |
Air density can be defined as the number of air molecules per unit volume. Near sea level there are about 2.7x1019 molecules per cm3(cubic centimeter) or 4.4x1020 molecules per inch3. While this may seem like a lot of molecules in a small area, molecules are very small. By comparison, the number density for solids and liquids is much higher. In a gas there is lots of empty space between the individual molecules.
Air molecules are held near the earth by gravity. In other words, air has weight. Weigh an empty bag, then fill it with air, it now weighs more.
The weight of all of the air above a given point in the atmosphere squeezes air molecules closer together, which causes their numbers in a given volume to increase (increase in number density). The more air above a level (and hence the more weight of air above a level), the greater the squeezing effect (or compression).
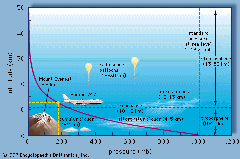
Since air density is the number of air molecules in a given space (volume), air density is typically greatest at the surface or sea level (where it is squeezed by the weight of the entire atmosphere) and decreases as we move up in the atmosphere because the weight of air above becomes less and hence there is less of a squeezing effect.
From a microscopic point of view, gas pressure is caused by the collisions of gas molecules on a surface. Each individual collision provides a tiny push (or force) on the surface that it contacts. The sum total of all of these tiny forces determines the air pressure. The physical units for pressure is force per area.
The weight of the air molecules acts as a force upon the earth. The amount of force excerted over an area of surface is called atmospheric pressure or air pressure. Near sea level, the average air pressure is about 14.7 pounds per square inch. In this class we will use the unit millibars(mb) to specify air pressure. At sea level the average air pressure is 1013 mb.
Since the air (a gas) is a fluid, the pressure force acts in all directions, not just downward. The pressure force pushing downward due to the weight of the air is the same as the pressure force acting sideways and even upward. If you are having trouble understanding this, make an analogy with another fluid liquid water. Consider a tall glass of water. The water pressure anywhere in the glass depends on the weight of the water above (that is the deeper you move downward in the glass, the stronger the water pressure.) The pressure force is not just downward because if you make a hole in the side of the glass water is forced out by the water pressure.
In the atmosphere, the air pressure at any point depends on the weight per area of the air above that point. As we climb in elevation, fewer air molecules are above us; hence, atmospheric pressure always decreases as you move upward in the atmosphere. Another way to look at it is that the air pressure at any point in the atmosphere is exactly enough to support the weight of the column of air above it. A balance exists between the gravitational force pushing air downward and the pressure force.