Fri., Mar. 31, 2006
Today
we'll
turn our attention to the formation of precipitation in clouds.
You find this discussed in the second half of Chapter 5 in the textbook.
Only two of the ten clouds types (nimbostratus and cumulonimbus) are
able to produce significant
amounts of precipitation. It is not that easy to turn small water
droplets or ice crystals into much larger precipitation particles such
as raindrops.
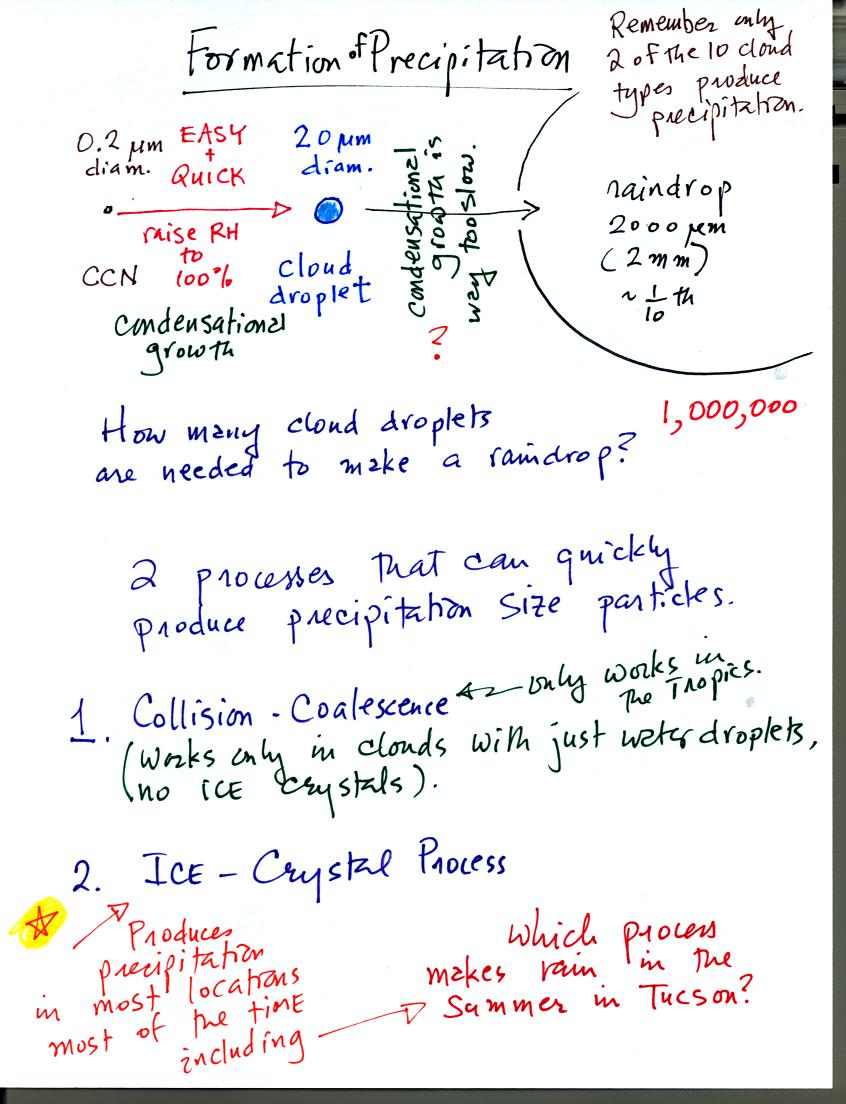
The upper part of the figure shows typical sizes of cloud
condensation nuclei (CCN), cloud droplets, and raindrops. As we
saw in the cloud in a bottle demonstration it is relatively easy to
make cloud droplets. You raise the RH to 100% and water vapor
condenses pretty much instantaneously to form a cloud droplet. It
would take much longer for condensation to turn a cloud droplet into a
raindrop. Part of the problem is that it takes about 1 million
cloud droplets of water to make a raindrop.
There are two processes capable of quickly producing precipitation
sized particles in a cloud.
The collision coalescence process works in clouds that are
composed on water droplets only. Clouds like this are found in
the tropics. We'll see that this is a pretty easy process to
understand.
The ice crystal process produces precipitation in most locations at
most times of the year. This is the process that makes rain in
Tucson, even in the hottest part of the summer.
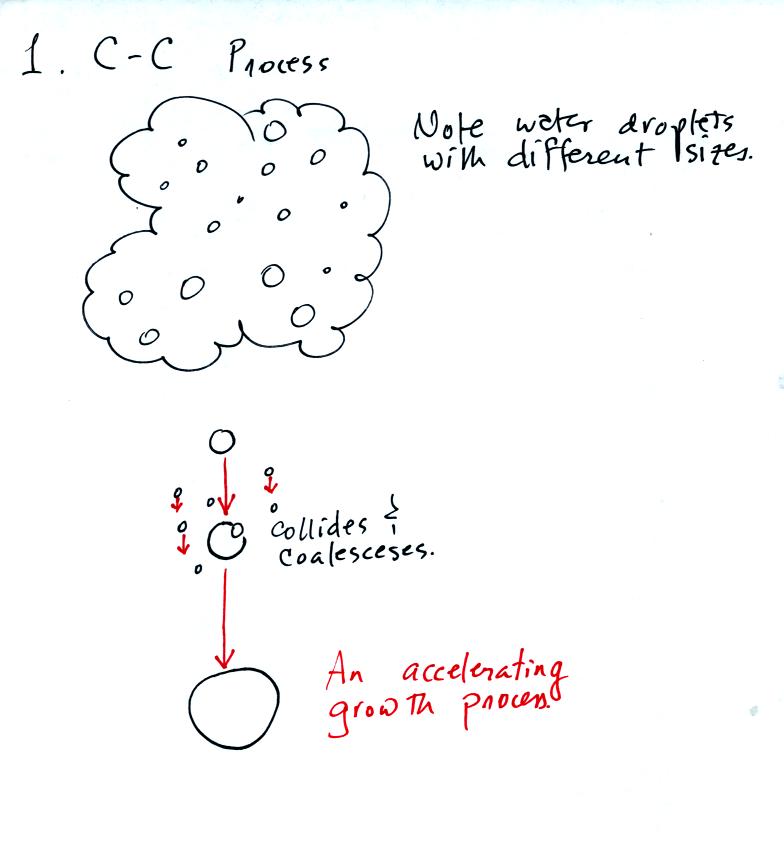
The collision coalescence process works best in a cloud
filled with cloud droplets of different sizes. As we saw in a
short video the larger droplets fall
faster than the small droplets. The large droplets overtake and
collide with the smaller ones. The droplets then stick together and
form any even larger droplet that will fall faster than before and
sweep out a larger volume. In this accelerating growth process an
above averaged sized droplet can
quickly turn into a raindrop.
The raindrops that fall from nimbostratus clouds tend to be
smaller
than the raindrops that fall from cumulonimbus clouds. The
growing raindrops don't spend as much time in the Ns cloud because the
cloud is thin and the updrafts are weak.
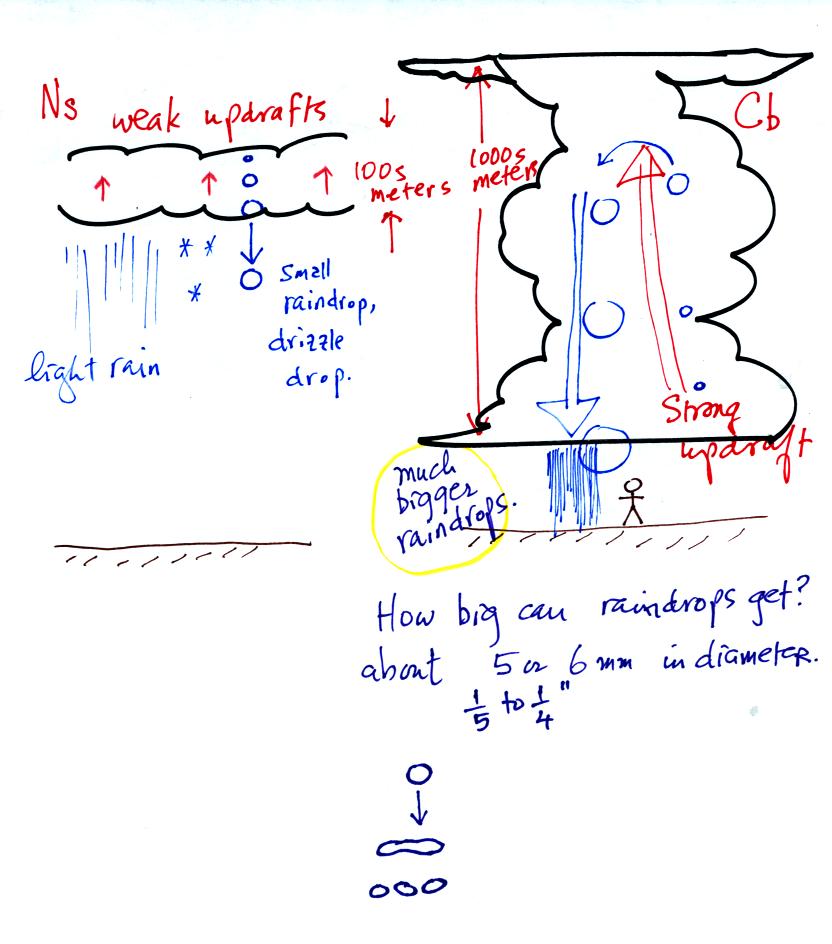
Note raindrops can grow to 5 or 6 mm (1/4 inch) in
diameter. The
wind resistance that a large drop encounters as it falls through a
cloud causes it to flatten out, start to flop around or wiggle, and
eventually break into smaller pieces.
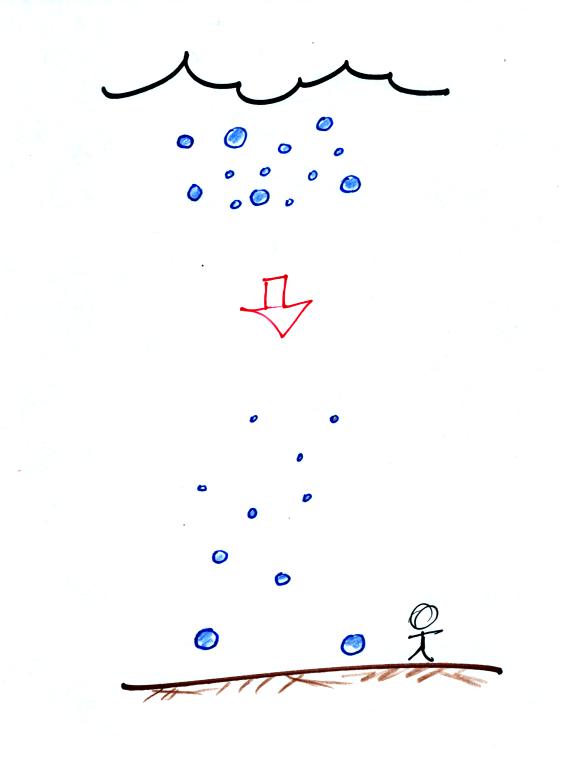
You may have noticed a few what seem to be very large raindrops hitting
the ground with an impressive splot at the beginning of a summer
thunderstorm. The figure below is one
explanation of this phenomenon.
Now before learning about how the ice crystal process we
need to look at the structure of cold clouds (clouds which contain ice
crystals and water droplets)
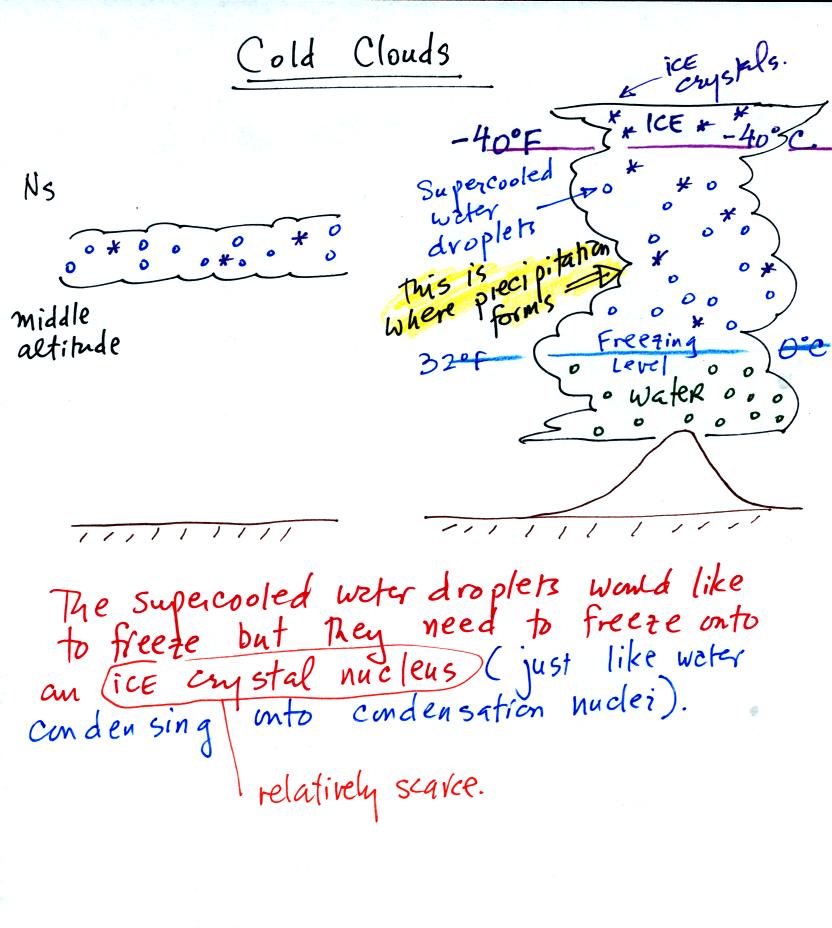
A large part of thunderstorm clouds and all of nimbostratus clouds
are composed of a mixture of supercooled water droplets (water that has
been cooled to below freezing but hasn't froze) and ice crystals.
This is called the mixed phase
region. This is where the ice crystal process will produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
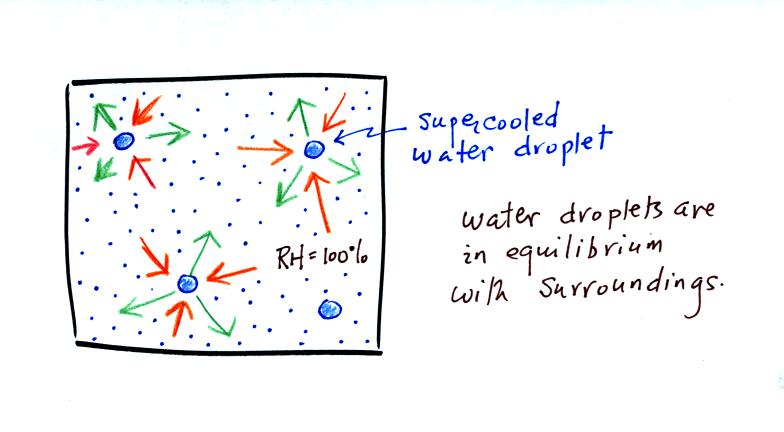
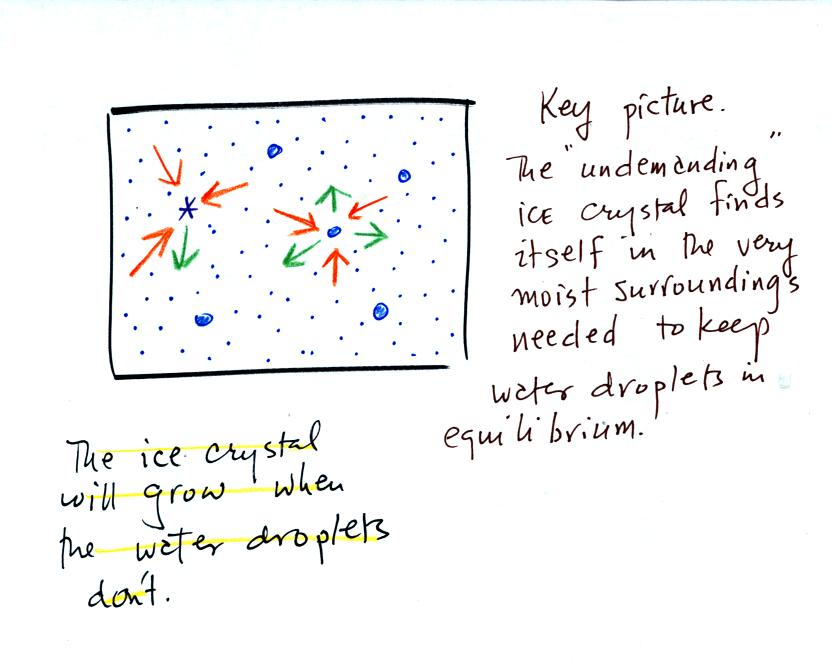
The supercooled water droplets are in equilibrium with their
surroundings. Otherwise the cloud wouldn't be there, it would
evaporate away. This means the surrounding air must be saturated
(RH=100%) in order to supply enough condensation to balance out
evaporation from the droplets. The little "specks" in the picture
represent water vapor. Note the higher water vapor concentration
above compared to the picture below.
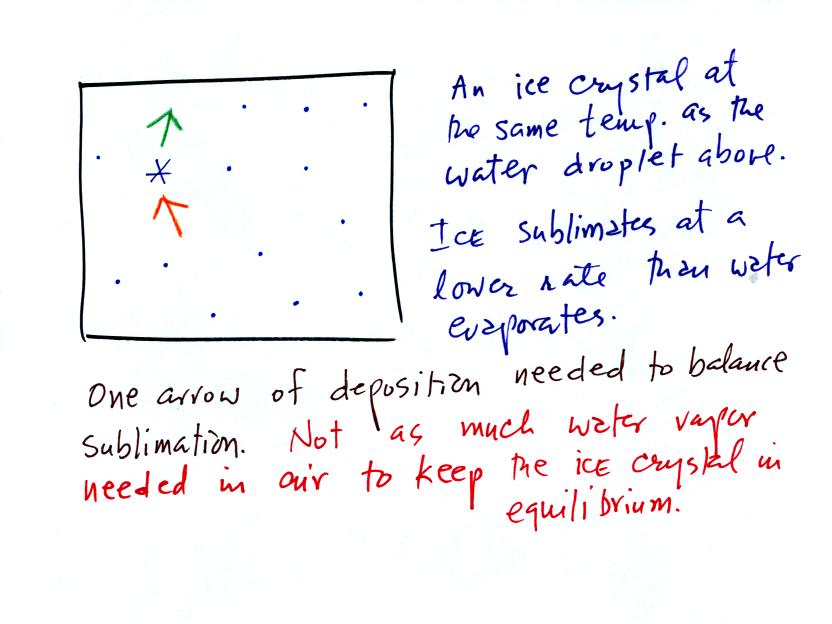
It takes more energy to break bonds in an ice crystal and turn
into water vapor than to change from water to water vapor. An ice
crystal at the same temperature as a water droplet won't sublimate away
as quickly as a water droplet evaporates. Less moisture is
required in the surrounding air to keep an ice crystal in equilibrium.
This is illustrated in the following analogy
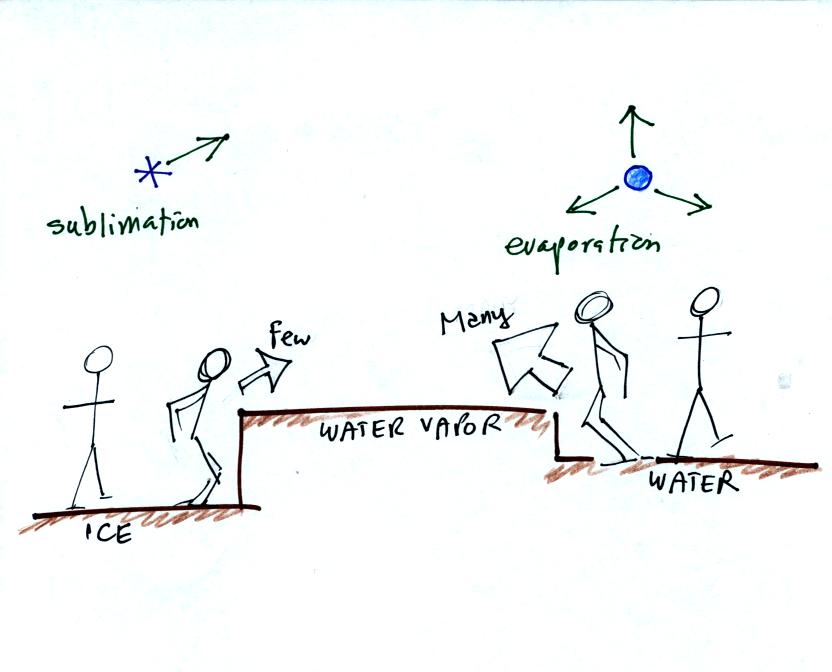
Fewer people are able to make the 2 or 3 foot jump than people able to
make the 1 foot jump.
More water vapor is being deposited onto the ice crystal is losing
by sublimation. The ice crystal will grow, the water droplets are
in equilibrium and won't change size.
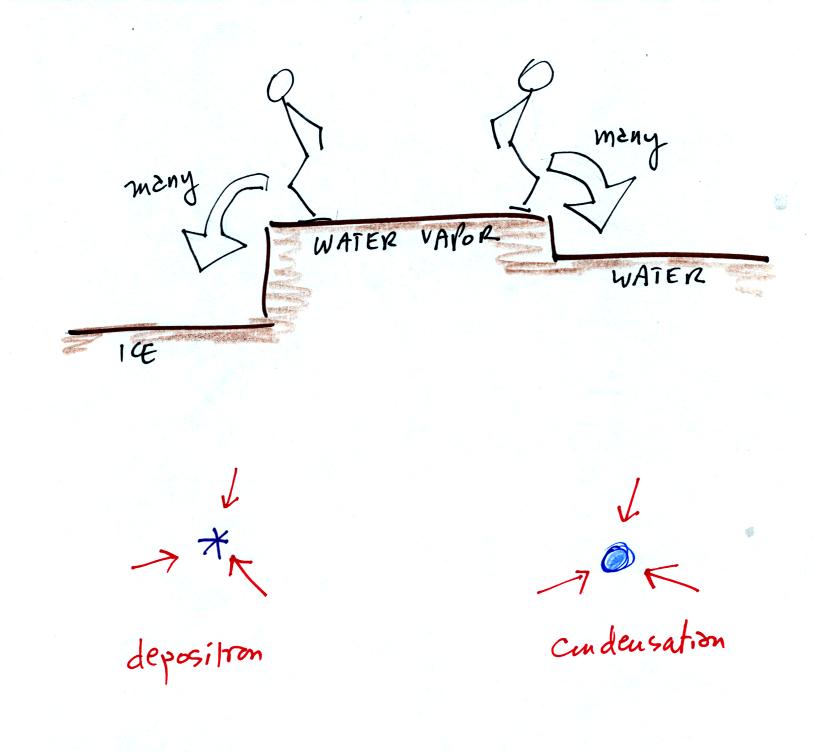
The rates of deposition and condensation depend on the amount of
water vapor surrounding the ice crystal and water droplet. Since the
same amount of water surrounds both particles, the rates of deposition
and condensation will be equal.









