Monday Mar. 27, 2006
The Experiment #3 reports have been graded and were returned in
class. Revised reports are due Mon. Apr. 11. Please return
the original report when you turn in a revised report.
The Optional Assignment was collected in class and a handout with
answers was passed out.
John Keller, the PhD student from the Lunar and Planetary Lab,
conducted a follow up survey on student understanding of the greenhouse
effect during the first 15 minutes of class.
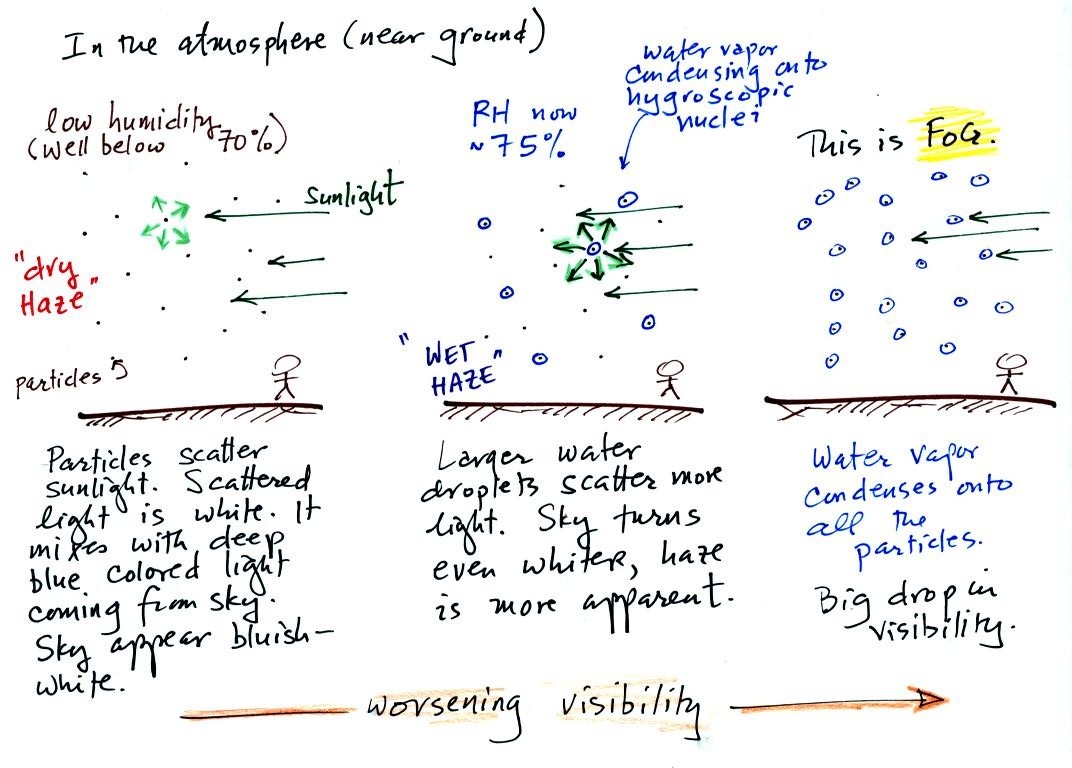
This is a larger version of the figure drawn in class (bottom of
p. 91 in the photocopied notes). This figures shows how cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter sunlight and
change the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze."
If you were to drive from the dry Southwestern US into the humid
Southeastern US one of the first things you would notice is the hazier
appearance of the air. Because the relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze."
Finally when the relative humidity increases to 100% fog forms.
Fog can cause a severe drop in the visibility.
Next we
attempted to make a cloud inside a bottle
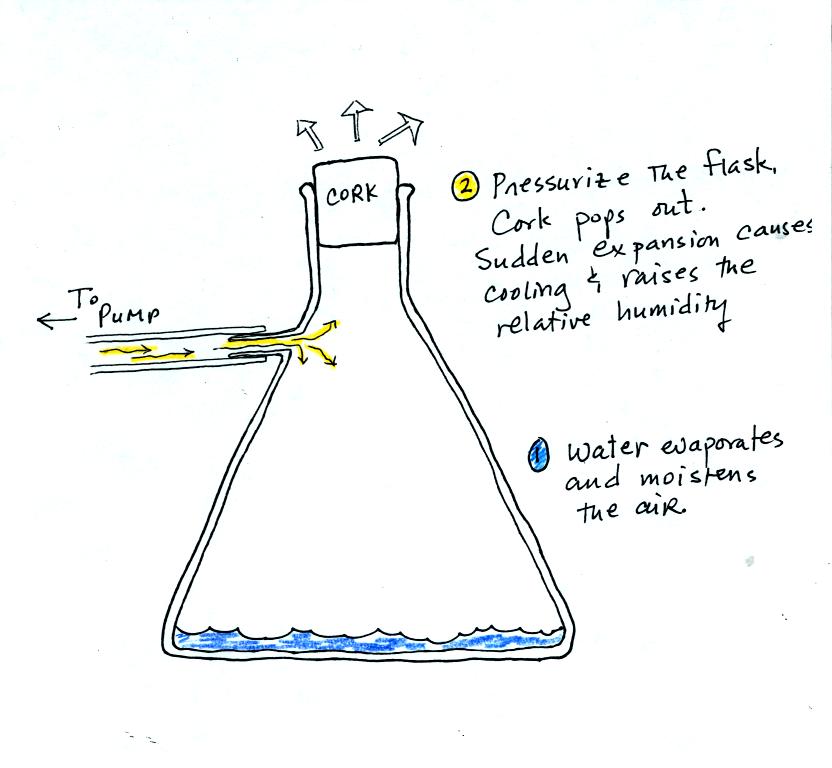
We used a strong thick-walled 4 liter flask. There was a little
water in the bottom of the flask to moisten the air in the flask.
Next we pressurized the air in the flask. At some point the
pressure blows the cork out of the top of the flask. The air in
the flask expands outward and cools. This cooling increases the
relative humidity of the moist air in the flask to 100% (probably more
than 100%) and water vapor condenses onto cloud condensation nuclei in
the air. A thin cloud became visible at this point. The
cloud droplets are too small to be seen with the human eye. You
can see the cloud because the water droplets scatter light.
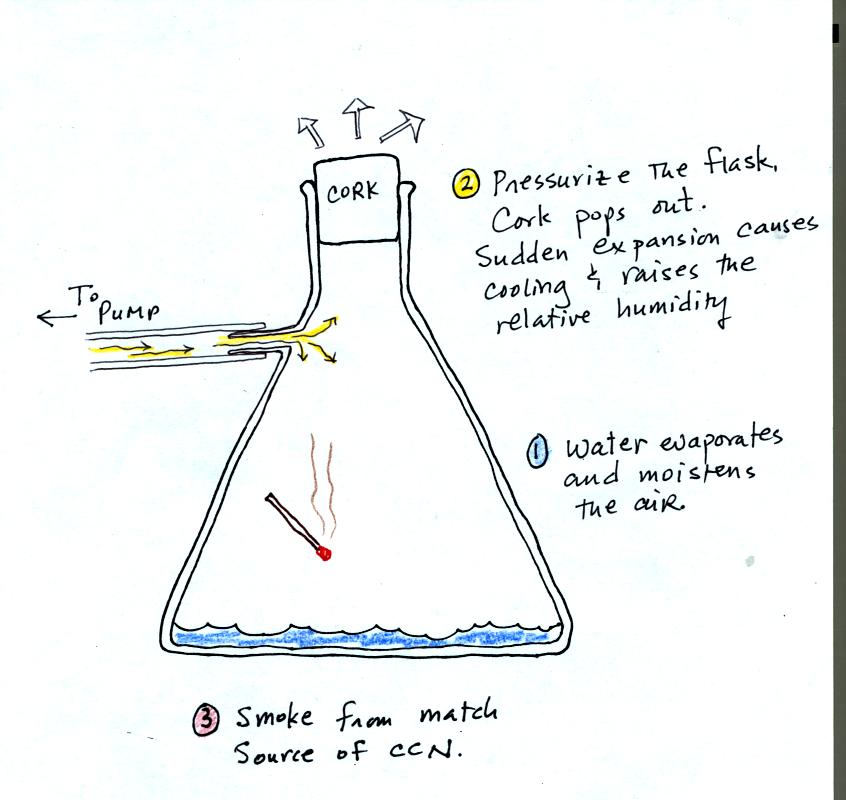
The demonstration was repeated a second time (perhaps a third time)
with one small change. A burning match was dropped into the
bottle. The smoke from the match consisted of lots of very small
particles that act as condensation nuclei. The cloud that formed
this time was more visible and easier to see
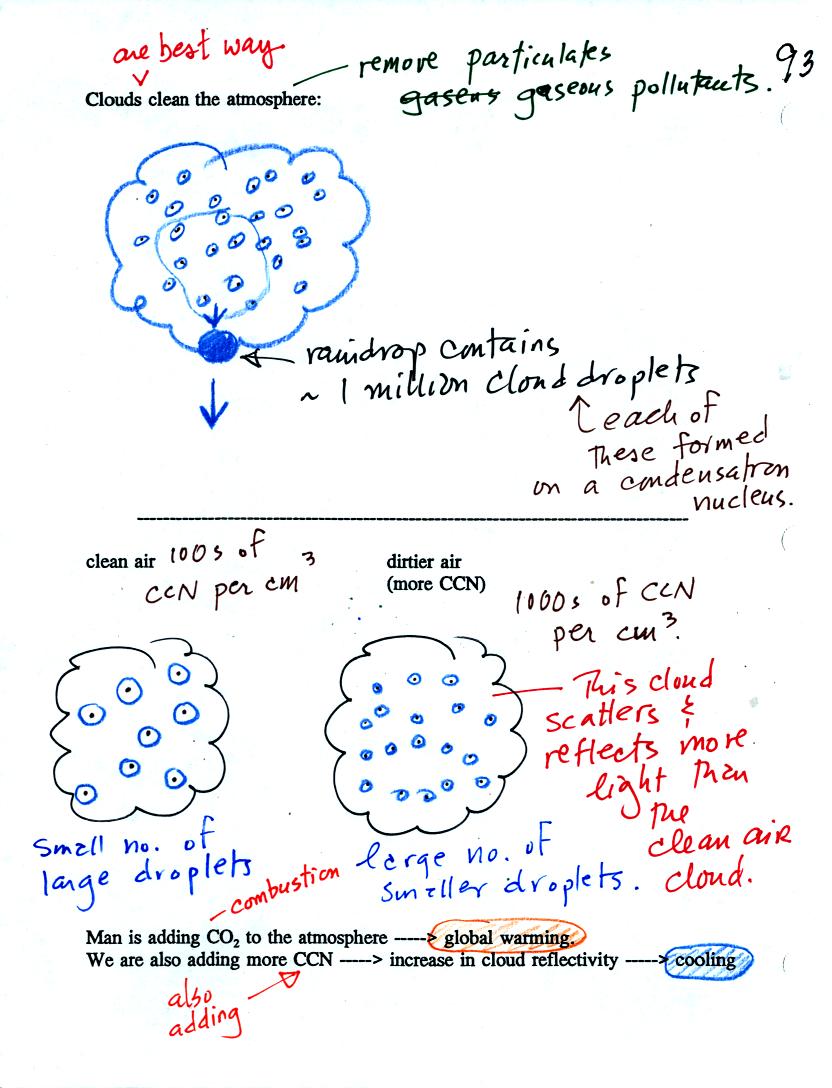
Clouds are one of the best ways of cleaning the atmosphere
(cloud
droplets form on particles, the droplets clump together to form a
raindrop, and the raindrop carries the particles to the ground).
Gaseous pollutants can dissolve in the water droplets and be carried to
the ground by rainfall also.
A cloud composed of a large number of small droplets is more reflective
than a cloud composed of a smaller number of larger droplets.
Combustion of fossil fuels adds carbon dioxide to the atmosphere but
also adds condensation nuclei. The increasing greenhouse gas
concentrations are expected to warm the earth. More particles
might make clouds more reflective which could cool the earth.
We were
just able to begin the next topic, identifying and classifying clouds,
before the end of class.

The ten main cloud names are listed above. Rather than just
memorizing the tens names it makes better sense to learn the 5 key
words and learn what they tell you about the 10 cloud types.




