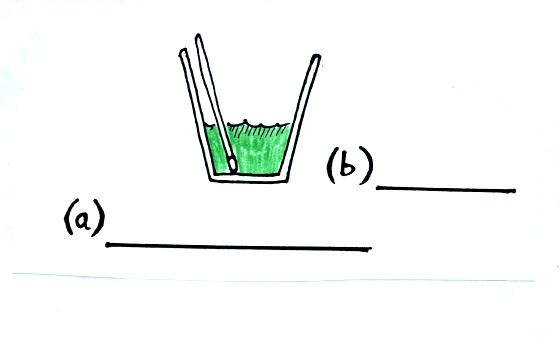
We first measure the mass and temperature of some water. The
water will be the source of the energy needed to evaporate liquid
nitrogen. By measuring the temperature of the water before and
after the liquid nitrogen is evaporated, we will be able to determine
how much energy was used.
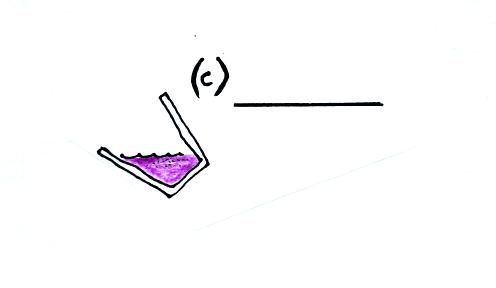
We also need to measure the mass of liquid nitrogen. We don't
need to worry about the temperature, it's -320 F. The liquid
nitrogen can't get any warmer than that and still remain a liquid.
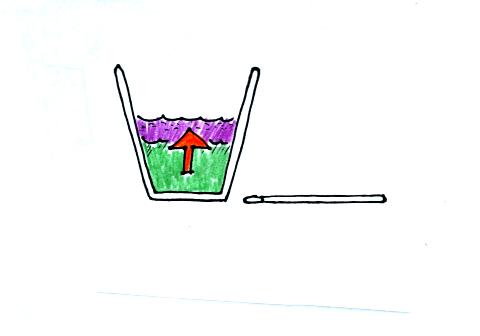
We pour the liquid nitrogen into the cup of water and wait.
Energy will flow from the warmer water into the very much colder liquid
nitrogen. We perform the experiment in a styrofoam cup and assume
that no energy is flowing into the air in the room (because the water
started out at room temperature there won't be much energy flowing from
the water into the room anyway).
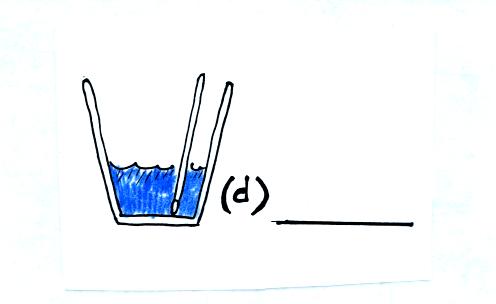
Now we remeasure the temperature of the water. The water should
be colder because some of its energy was used to evaporate the
nitrogen. We assume that the mass of the water has stayed the
same.



