Wed., Jan. 25, 2006
The Practice Quiz Study Guide (online
version) is now available.
The first Optional homework Assignment
was distributed in class. This assignment is due next Wed., Feb.
1.
Next we will start trying to understand air pressure. Air
pressure is important because small horizontal differences in air
pressure can
start the wind blowing (sometimes violently). Before tackling
pressure we will review mass, weight, and density.
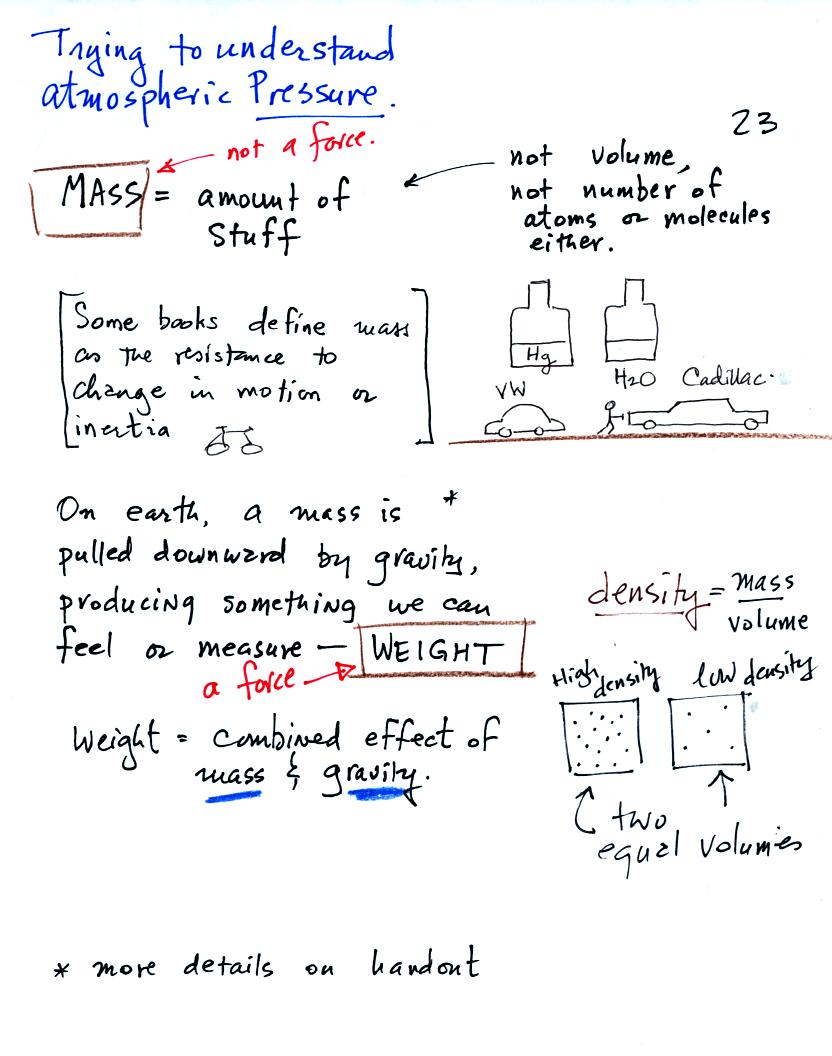
Mass is just the amount of material in an object. Mass is not the
same as volume - bottles containing equal volumes of water and mercury
were passed around class. The mercury has a lot more (13.6 times
more) mass than the water. A Cadillac has
more mass than a Volkswagen. It takes a lot for force to get a
stopped Cadillac moving than a stalled Volkswagen. It is harder
to win a sprint on a steel frame bicycle than with a bike with an
aluminum or carbon fiber frame.
Gravity acting on a mass produces weight. Weight is a force, mass
is not a force. We sometimes use mass and weight
interchangeably. We can do this because gravity on the surface of
the earth
doesn't change.
The
following information wasn't covered in class
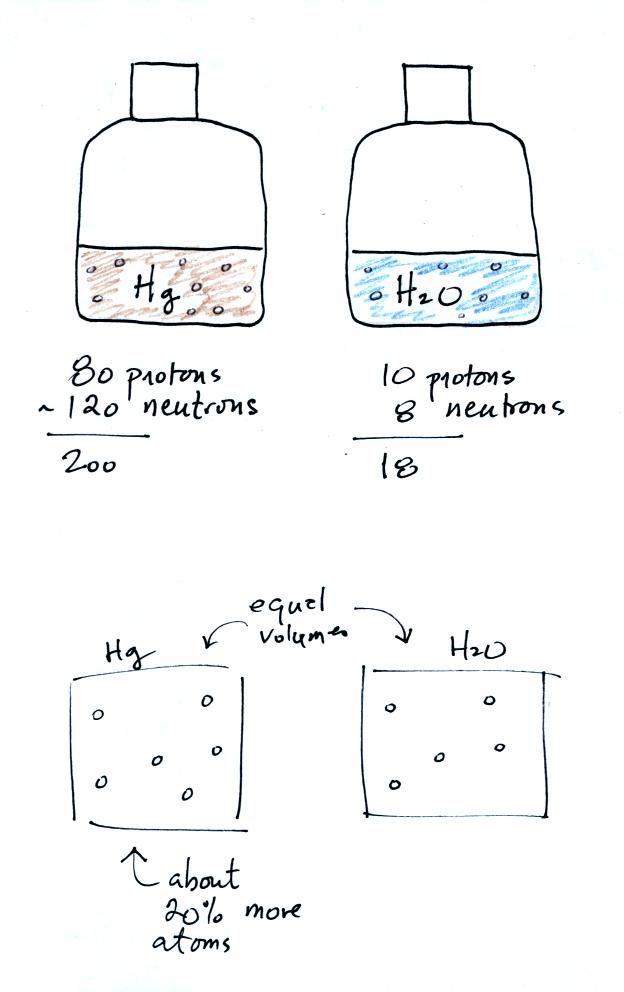
How can you explain the difference in the masses of mercury
and water?
Mercury has a density of 13.6 g/cm3, water a density of 1 g/cm3.
There is 13.6 more mass in the volume of mercury than in the same
volume of water.
The main reason for the difference is that a mercury atom has more
protons and neutrons than a water molecule.
Mercury has an atomic number of 80 which means there are 80 protons in
its nucleus. Mercury's atomic weight is about 200; mercury has 80
protons and about 120 neutrons (mercury comes in slightly different
forms or isotopes: some atoms have 121 or 122 neutrons others 118 or
119 neutrons, 120 is a nice average).
A water molecule consists of two hydrogen atoms with 1 proton each and
an oxygen atom with 8 protons and 8 neutrons. That gives an atomic
weight of 18.
If you divide 200 by 18 you get 11.1
That is not quite 13.6, so there must be a few more mercury atoms
squeezed into a volume than you would find in water. About 20%
more mercury atoms per cubic centimeter is about all you would need.
This is
the top half of a handout distributed in class.
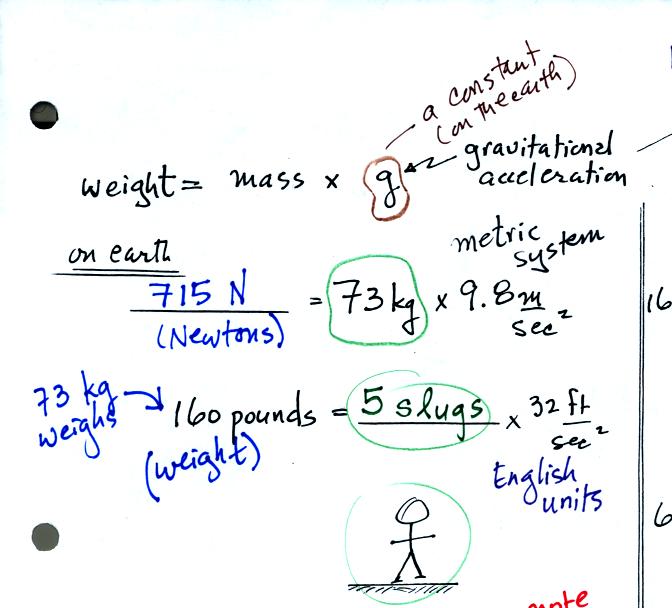
Weight is just mass multiplied by a constant, g, called the
gravitational
acceleration. On the earth g
is 9.8 m/sec2 or 32 ft/sec2.
The gravitation acceleration depends on the size (radius) and mass of
the earth.
You've probably heard of kilograms (kg) and pounds (lbs).
Kilograms are units of mass
in the metric system, pounds are English system units of weight. It is OK to
use them interchangeably on the earth because a mass of 73 kg will
always produce a weight of 160 pounds. Strictly speaking the
metric system units of weight are Newtons (the 73 kg mass has a weight
of
715 Newtons). The units of mass in the English system are
slugs. A 5 slug object (73 kg in the metric system) weights 160
pounds.
Here's the bottom half of the handout.
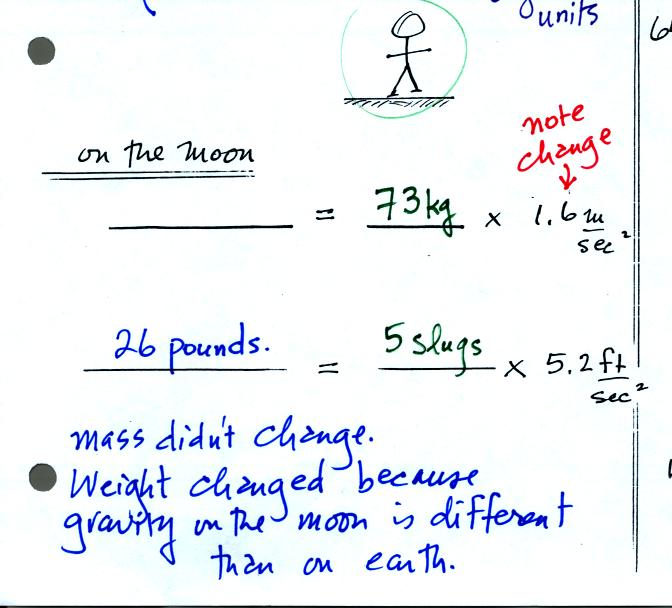
On the earth a person with a mass of 73 kg weighs 160
pounds. If
we travel to the moon however gravity will be different. On moon
the person will still have a mass of 73 kg or 5 slugs. The
person's weight however will be different because the value of the
gravitational constant will be different than on the earth (the moon is
smaller and has less mass than the earth). On the moon, a person
with a mass of 73 kg will only weigh 26 pounds.
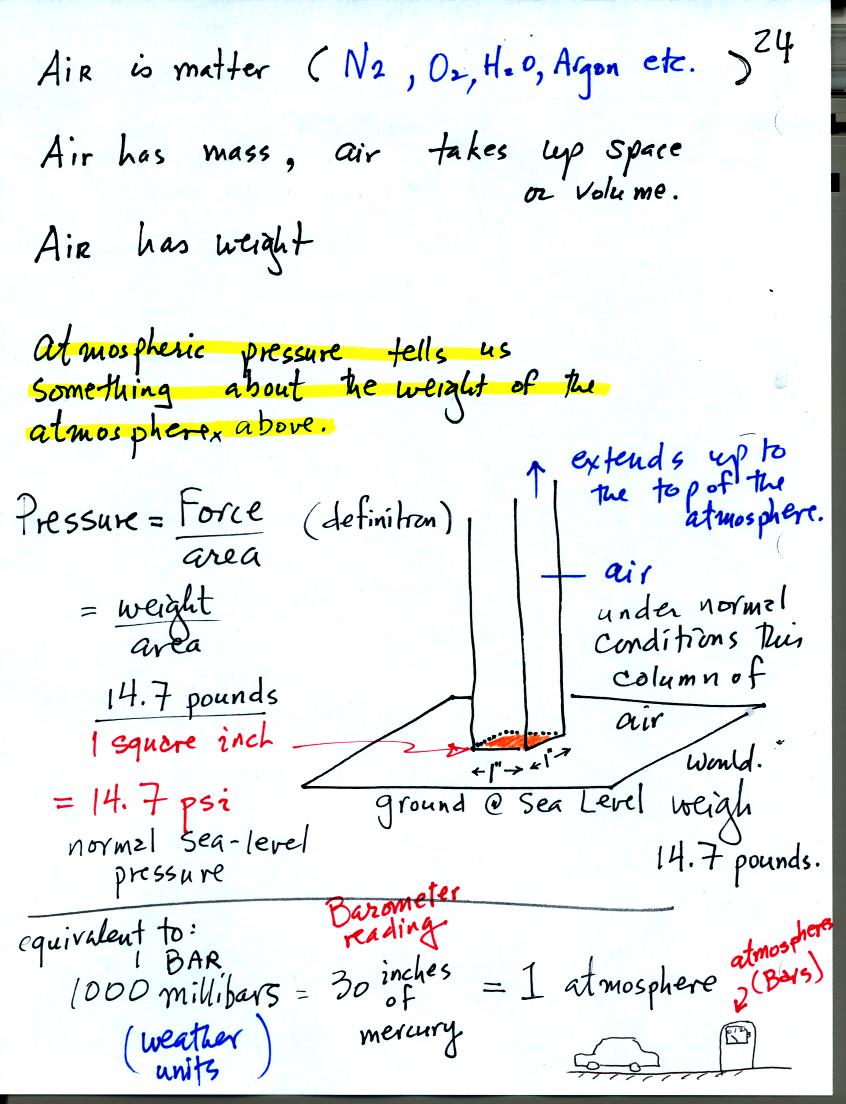
Gravity pulls downward on the air surrounding the earth producing
weight (people didn't really realize that air had mass and weight and
didn't devise ways of measuring air pressure until the
1600s). Air pressure is determined by and tells you something
about the weight of the air overhead. This is one way of trying
to understand atmospheric pressure.
A one inch by one inch column of air extending from sea level to the
top of the atmosphere would, under average conditions, weigh 14.7
pounds (the same as a 4 foot long iron bar that was passed around in
class). Pressure is defined as force divided by area, in this
case weight divided by area. So a typical sea level pressure
would be 14.7 pounds per square inch or psi. These are the same
units used when filling an automobile tire with air. You usually
put around 30 psi into car tires.
We will use millibar (mb) units in our course. Standard
atmospheric
pressure is about 1000 mb or 30 inches of mercury. The second
value refers to the reading from a mercury barometer. 1000
millibars is equal to 1 bar or 1 atmosphere.
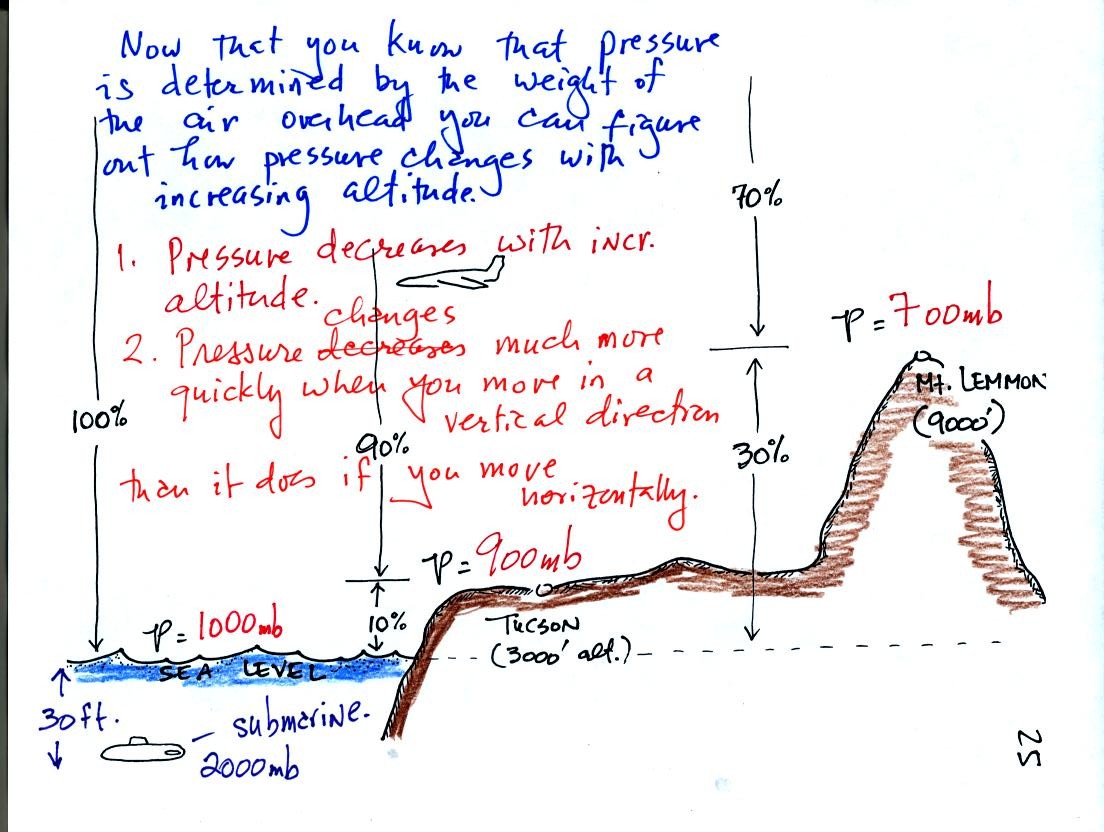
As you move upward through the atmosphere there is less and less air
left overhead. The pressure at any level in the atmosphere is
determined by the weight of the air remaining overhead. Thus pressure
decreases with increasing altitude. Pressure changes much more
quickly when you move in a vertical direction than it does when you
move horizontally. This will be important when we cover surface
weather maps. Meterologists attempt to map out small horizontal
changes or differences in pressure on weather maps. These small
changes are what cause the wind to blow and produce weather.
Pressure increases rapidly as you descend into the ocean. The
pressure at some level in the ocean is determined by the atmospheric
pressure plus the pressure produced by the weight of the water above
you. Water is much denser than air; pressure has doubled to 2000
mb when you are only about 30 feet deep in the ocean.
In the last few minutes of class we watched some more of the video tape
about Auguste Piccard. In this segment Auguste and his son
Jacques descended to 10,000 feet depth in the ocean in a
bathyscaph. At that depth the pressure is 5000 psi.
Jacques would later descend to 35,000 feet with another person.
That is as deep as you can go in the ocean.





