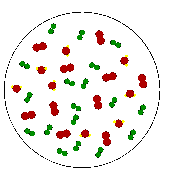
 A "Parcel of Air" is an iminaginary body of air about the size
of a large balloon that is used to explain the behavior of air.
We will describe what is meant by the relative humidity and dew
point temperature of air in a parcel. The parcel concept is used
because we often would like to know what will happen to air as it
moves in the atmosphere, and air tends to move together in blobs about
the size of parcels (not molecule by molecule). The parcel concept
will be extemely important in describing cloud and thunderstorm formation.
For one thing, we will want to keep track of the relative humidity in
an air parcels as it moves up and down in the atmosphere.
A "Parcel of Air" is an iminaginary body of air about the size
of a large balloon that is used to explain the behavior of air.
We will describe what is meant by the relative humidity and dew
point temperature of air in a parcel. The parcel concept is used
because we often would like to know what will happen to air as it
moves in the atmosphere, and air tends to move together in blobs about
the size of parcels (not molecule by molecule). The parcel concept
will be extemely important in describing cloud and thunderstorm formation.
For one thing, we will want to keep track of the relative humidity in
an air parcels as it moves up and down in the atmosphere.
The relative humidity in an air parcel is defined as the ratio of the amount of water vapor actually in the air to the maximum amount of water vapor required for saturation at a particular temperature
| water vapor content | ||
| Relative humidity | = | |
| water vapor capacity |
For example, air with a 50 percent relative humidity actually contains one-half the amount of water vapor required for saturation.
Air with a 100 percent relative humidity is said to be saturated because it is filled to capacity with water vapor.
One way to compute relative humidity is to take the ratio of the actual vapor pressure in a parcel (measured) with the saturation vapor pressure at the parcel temperature, or RH = e/es. However, following meteorological convention, we will use something called mixing ratio instead of vapor pressure. The definitions of mixing ratio, saturation mixing ratio, and its relationship to relative humidity will be provided in class and described in a handout.
Most people use Relative humidity to describe the water vapor content of the air, but it is widely misunderstood. Relative humdity in itself does not indicate the actual amount of water vapor in the air because it depends on temperature. For example, an air parcel at a temperature of 10°C with RH = 100% contains the same concentration of water vapor as an air parcel at a temperature of 20°C with RH = 50%. You should be able to convince yourself of this by using the Table 4-1 from the class handout.
The dew point temperature represents the temperature to which air would have to be cooled (with no change in moisture content) for saturation to occur. If you know the actual mixing ratio in an air parcel, you can use table 4-1 to get the dew point temperature.
The dew point temperature does indicate the actual vapor content of the air: the higher the dew point, the more water vapor in the air.
The difference between air temperature and dew point temperature can indicate whether the relative humidity is low or high.
Near the ground surface, we are often interested in how much net evaporation (evaporation minus condensation) will occur. The rate of net evaporation depends on:
Usually, unless a weather front passes, the dew point temperature will not change much during the course of the day. Assuming that the dew point temperature does not change during the day, what time of day should you water outside plants to best conserve water? ANSWER: In the morning around the time of the low temperature. This is typically when the relative humidity will be the highest. When relative humidity is high, the net rate of evaporation will be the slowest, allowing more liquid water to be absorbed into the soil and plants rather than evaporating.
Normally, near the Earth's surface, the relative humidity is less than 100%, therefore, net evaporation is occurring. To convince yourself, leave out an uncovered glass of water. The water will eventually evaporate. Once evaporated from the surface, water vapor moves around with the rest of the air. Occasionally, there may be net condensation occurring near the Earth's surface. If air near the ground is cooled below its original dew point temerature, fog will result. Fog is nothing more than a cloud at ground level. We will talk more about clouds next week.
Beside fog, are there ever situations near the Earth's surface when net condensation occurs? ANSWER: Yes, dew and frost.
When an object becomes colder than the dew point temperature, the air in contact with that object becomes colder than the dew point temperature, resulting in net condensation:
The dew point temperature is also used to determine the start date of the Tucson summer monsoon. A monsoon day is defined as an average of 24 hourly dewpoint values that is 55°F or greater. The start of the monsoon is defined as three consecutive days of the daily dewpoint average being 55°F or greater.
A couple of links: current dew point temperature and relative humidity on the Univ. of Arizona campus.
U.S. map of current dew-point temperature.