The recycling of elements among the various components of the Earth system is key to the continued functioning of the Earth as a living planet. Although many elements are critical, none is more central to the workings of the Earth system than carbon. Nearly all life (at least on Earth) is based on carbon. In fact carbon makes up about 50% of our dry weight (excluding water). Ecologists study ecosystems by tracking carbon exchanges through the system. For example, land plants take carbon dioxide out of the atmosphere in order to grow, while animals put that carbon back into the atmosphere when they eat the plants. In the ecosystem model, plants are the producers of food, while animals are the consumers. Both biological and physical processes are involved in the recycling of carbon, and they are so closely intertwined that it is difficult to separate the two. In this class we will do no more than look at a simplified overview of the carbon cycle focusing mainly on the atmospheric reservoir of carbon and how it is changing in response to human activities.
Beside being the central constituent of all organic compounds, carbon in the atmosphere, which is mostly in the form carbon dioxide, is an important greenhouse gas. Without the greenhouse effect of carbon dioxide in the atmosphere, life as we know it would not exist on Earth because the surface temperatures would be too cold. However, the actions of man have disrupted the natural carbon cycling by causing imbalances in the flow of carbon to and from the atmospheric reservoir. The net effect has been a sharp increase in the amount of carbon dioxide stored in the atmosphere. The biggest concern with this is how will the extra carbon dioxide affect the Earth's climate and in turn its life? As we will see this is a very difficult question to answer due to the complexities of both the carbon cycle and the climate system, which are interlinked. The ultimate outcomes stemming from anthropogenic perturbations to the carbon cycle remain quite uncertain. Thus, what, if anything, should be done about the human-caused increases in the carbon dioxide concentrations in the atmosphere remains a hotly debated issue.
| Simplified preindustrial carbon budget diagram. Numbers associated with reservoirs are in units of Petagrams (1015 grams) of carbon stored. Numbers associated with fluxes are in units of Petagrams per year. |
The diagram above is an estimate for the carbon cycle that existed prior to humans adding significant carbon dioxide to the atmosphere. The boxes are reservoirs for carbon. Reserviors are places where carbon is stored. You should notice the relative sizes of the reservoirs. The largest reservoir is the lithosphere, which includes buried material such as rocks and the fossil fuels. The smallest reservoir is the atmosphere. The arrows on the diagram indicate fluxes (or movement) of carbon between reservoirs. Each flux arrow has a sink and a source component. A sink is a removal mechanism and takes carbon out of a reservoir and a source is a mechanism that adds carbon to a reservoir. For example, respiration and decay is a sink (removal mechanism) for carbon stored in the land biota and soils (life on land) and a source for carbon stored in the atmosphere.
Notice that the fluxes of carbon into and out of the atmosphere each year are equal to one-fourth of the total amount of carbon dioxide stored in the atmosphere. Thus, the turnover rate for carbon in the atmosphere is quite fast. You should understand the fluxes between the land biota and atmosphere. Photosynthesis, which is carried out by algae and terrestrial green plants, removes carbon from the atmosphere (sink for carbon stored in atmospheric reservior) and places it in the land biota (source for carbon stored in the land biota). Plants grow by building structures with this carbon. The basic chemical equation below shows that carbon dioxide from the atmosphere is used to create carbohydrates (hydrated carbons).
| Photosynthesis: CO2 + H2O + Sunlight → C(H2O) + O2 |
The reverse flux is respiration and decay. All animals return CO2 directly to the atmosphere as a by-product of their respiration. The carbon present in animal wastes and in the bodies of all organisms is released as CO2 by decay or decomposer organisms (chiefly bacteria and fungi) in a series of microbial transformations. The basic chemical equation below shows that when carbohydrates are consumed, the carbon is moved back to the atmosphere in the form of carbon dioxide.
| Respiration and Decay: C(H2O) + O2 → CO2 + H2O + Energy |
Carbon dioxide is continuously exchanged between the atmosphere and the ocean. The distribution of sources and sinks of CO2 is tied to the circulation and productivity patterns of the oceans. In regions of the ocean where there are high rates of primary productivity (building of organic material through photosynthesis), there is a net flux of carbon from the atmosphere into the oceans. Conversely, in upwelling regions where the ocean water is CO2 rich, the net flux of carbon is from the oceans into the atmosphere. Before the carbon cycle was disturbed by human activities, the flux of CO2 into the ocean was probably closely balanced by the flux out. Notice that the carbon fluxes between the ocean and the atmosphere are larger than between the atmosphere and land biota.
Part of the organic carbon--the remains of organisms--has accumulated in the Earth's crust as fossil fuels (e.g., coal, gas, and petroleum), limestone, and coral. The flux linkages between the atmosphere and lithosphere were probably closely balanced before the industrial revolution. Note how slow these processes are (small fluxes) compared withe exchanges of carbon between the atmosphere and oceans or land.
After 1850, humans began to significantly alter the balanced carbon cycle through burning fossil fuels and deforestation. The carbon stored in fossil fuels is now being released in vast amounts as CO2 when these fossil fuels are "burned" or used as energy sources. Deforestation adds carbon dioxide to the atmosphere in two ways: (1)Trees that have been removed, no longer participate in photosynthesis, which means they no longer remove CO2 from the atmosphere ;and (2)When these trees are burned or decay, they release all the stored carbon they contain. The processes of fossil fuel burning and deforestation are shown in bold on the pre-industrial carbon budget diagram below. Notice that the amount of carbon stored in the atmosphere is now 859 Petagrams (401 ppm), which is 43% greater than the 600 Petagrams (280 ppm) that was contained in the atmosphere prior to the industrial revolution. As expected, the burning of fossil fuels and deforestation are causing the amount of carbon dioxide in the atmosphere to increase. Another change noted in bold is the increase in carbon flux from the atmosphere to the oceans by 2.4 Petagrams per year. Thus, the oceans are in effect storing some of the carbon dioxide released to the atmosphere by human activity. The missing sink, which is described in the next section of text, represents an unidentified process that is also removing carbon dioxide from the atmospheric reservoir; however, which reservoir (or combination of reservoirs) is storing this carbon is unknown.
| Simplified postindustrial carbon budget diagram. Numbers as in preindustrial figure above. The bold numbers represent values that have changed since the industrial revolution. |
We can fairly well compute the rate of carbon release to the atmosphere by fossil fuel burning and deforestation. And if these were the only changes to the carbon budget, we could easily calculate how fast the atmospheric reservoir of carbon would increase. However, the system itself (and any intertwined systems) responds to these human perturbations. We do not understand the details and complexities of the carbon cycle well enough to accurately predict how the various fluxes and reservoirs will change with time as humans continue to add CO2 to the atmosphere. One consequence of this is the missing sink for atmospheric carbon dioxide, which is described in the section below.
One thing that we do know for sure is that the concentration of CO2 in the atmosphere is increasing. We have very accurate measurements taken at Mauna Loa, HI since the late 1950s. Prior to that we can estimate the atmospheric CO2 concentrations from ice core samples. Before the Industrial Revolution, the concentration of CO2 in the atmosphere was about 280 parts per million (ppm) and today it is about 401 ppm (see figures below).
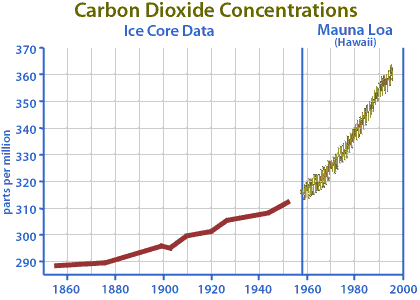
|
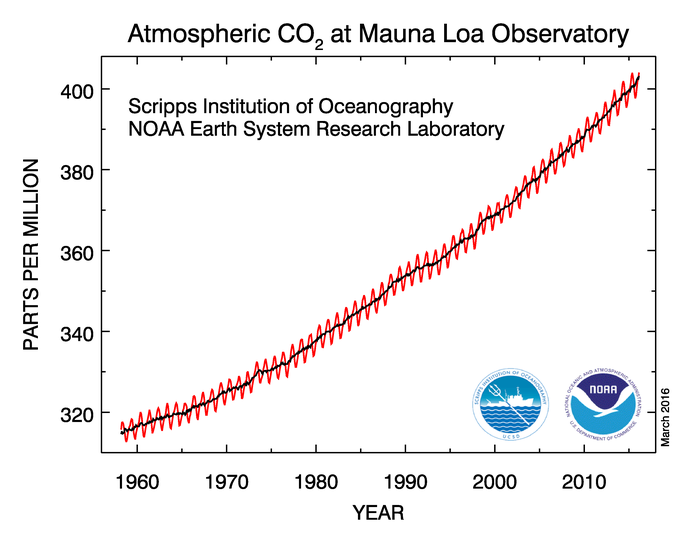
|
| Close-up Atmospheric CO2 concentration from Mauna Loa |
There is no doubt that CO2 levels in the atmosphere are increasing, and the primary reason it is increasing is due to human activities. The black line in the figure above highlights the observed trend of increasing CO2 in the atmosphere. The year to year fluctuations shown in red are tied to the seasonal cycle of plant growth in the Northern Hemisphere's terrestrial ecosystems. In the Northern hemisphere summer, land plants grow by photosynthesis, taking in lots CO2, which reduces the CO2 in the atmosphere. Thus, the minimum CO2 concentration each year happens at the end of the growing season in late summer and early fall. Conversely, in the Northern hemisphere winter, photosynthetic activity decreases, but the processes of respiration and decay continue, thus CO2 levels in the atmosphere increase. The maximum CO2concentration each year happens just prior to the growing season in early spring.
Scientists have developed models of the carbon cycle to make predictions of how the system will respond to the human perturbations. One response that we are reasonably sure about is that the oceanic uptake of CO2 has increased in response to the increase in the atmospheric concentrations of CO2. In other words as CO2 accumulates in the atmosphere, some of it is absorbed into the ocean water. Physically some of the carbon dioxide gas in the atmosphere dissolves in ocean water. This is shown as flux of 2.4 Petagrams carbon per year in bold print in the postindustrial carbon budget diagram. However, there remains a problem: Our models of the carbon cycle predict that the present day concentration CO2 in the atmosphere should be about 482 ppm, but the measured amount is 401 ppm; therefore, more CO2 is being removed from the atmosphere than is predicted. This discrepancy has been called the "missing sink" problem for atmospheric CO2. It is "missing" because we have not identified it yet and it is a "sink" for carbon stored in the atmosphere because it removes carbon from the atmosphere. The flux of CO2 out of the atmosphere due to the missing sink is about the same as the additional flux into the oceans that has come about in response to the human activities that release CO2 into the atmosphere.
For the decade of the 1990s, the global carbon cycle can be summarized as follows (units are Petagrams of carbon. A petagram is one billion metric tonnes = 1000 x one billion kg):
| Atmospheric increase | = | Emissions from Fossil fuels | + | Net emissions from changes in land use | - | Oceanic uptake | - | "Missing" carbon sink |
| 3.2 (±0.2) | = | 6.3 (±0.4) | + | 2.2 (±0.8) | - | 2.4 (±0.7) | - | 2.9 (±1.1) |
Attention on the global carbon cycle over more than 25 years has focused on the apparent imbalance in the carbon budget in the above equation -- the so-called "missing sink." Thus, the observed accumulation of carbon in the atmosphere has actually been less than expected. Breaking down the numbers given above we can say: Of all the CO2 added to the atmosphere by human activity, roughly 40% remains in the atmosphere, 30% is absorbed by the oceans, and the remaining 30% is the "missing sink". Perhaps surprisingly, those percentages seem to remain roughly the same, regardless of fluctuations in the amount of CO2 that is added to the atmosphere by human activities.
The problem of the missing CO2 sink has still not been satisfactorily explained. Where is this "missing" carbon going? The most accepted explanation, though not scientifically proven, is that much of the missing carbon is being taken up and stored in land plants. It appears that photosynthesis has been faster than decomposition for decades although we are still unable to say for sure exactly where the carbon is stored. There are several possible contributing factors. One is that plants may be growing faster due to CO2 fertilization. Another possibility is related to global warming. Global average temperatures have increased (especially since the 1980s). This seems to have extended the growing season in northern forests. The longer growing season could allow trees to take in more CO2. Regrowth of natural forests after agricultural abandonment, particularly assoicated with small family farms, may be removing more CO2 than thought. Some also point to suppression of forest fires as a possible contributing factor in keeping CO2 out of the atmosphere. These explanations seem reasonable considering that forests contain about 90% of all of the living biomass on land. Those of you who have followed the global warming debate closely, know that part of the Kyoto Treaty contains "carbon credits" for countries that plant new trees that should effectively remove CO2 from the atmosphere.
The carbon cycle on Earth is very complex and there is much that we do not understand about its operation. Regardless of the ultimate effects that increased carbon dioxide will have on climate change, we have trouble understanding what happens to the carbon dioxide released by human activity. Currently the missing sink is acting to remove carbon dioxide from the atmosphere, which will reduce climate changes related to human emissions of CO2. Given that we do not understand this missing sink, there is concern that this sink will slow down or even stop in the future. If this happens, CO2 concentrations will begin to rise more quickly, perhaps resulting in more severe climate changes. However, in a warmer world with more CO2 in the atmosphere, one could argue that plants will thrive and take in lots of CO2.
There are several issues related to the oceans. As ocean water warms, it is not able to contain as much dissolved CO2. This is similar to what happens when you warm up a carbonated beverage. This effect means that warmer oceans will release CO2 into the atmosphere, which may accelerate further warming. There are questions as to how marine life will respond to higher amounts of carbon dioxide in the atmosphere and the ocean waters, particularly organisms that make carbonate shells. Higher amounts of dissolved CO2 in the ocean will lower the pH or make the oceans more acidic. There is much debate about how much ocean pH will change in response to higher CO2 levels in the atmosphere as well as how that will affect ocean life.
There are vast amounts of carbon stored in frozen materials on Earth, especially in the Arctic. Warming of this region could result in the thawing of permafrost soils and frozen methane, which will cause the release of carbon dioxide and methane into the atmosphere, and perhaps accelerate global warming and associated climate changes. A very good set of short videos on the carbon cycle and associated climate issues is available from the National Science Foundation's special report on the Carbon Cycle.
Finally, keep this in mind as we discuss the global warming issue: One of the many uncertainties in the prediction of future global warming is our poor understanding of the carbon cycle itself. Even though we know how much CO2 is being added to the atmosphere by fossil fuel burning, we are not able to predict with certainty what happens to all of it. Of course, this limits our ability to predict how future climate may change due to human emissions of CO2.
The Random House Dictionary defines a pollutant as "any substance, as certain chemicals or waste products, that renders the air, soil, water, or other natural resource harmful or unsuitable for a specific purpose." In 2007, the United States Supreme Court Decided that CO2 is a pollutant. However, since CO2 is not directly harmful to breathe, even at concentrations over 100 times greater than the current atmospheric concentrations (which incidentally is the approximate CO2 concentration in our exhaled breath), the only way the Supreme Court can make that ruling is to say that the potential climate changes that may come about due to CO2 will render the Earth harmful or unsuitable for people and other life. However, we do not understand the Earth's climate system well enough to make that prediction. The court's ruling probably has more to do with giving government the authority to regulate CO2 emissions. You may well agree with that, but it is misleading to classify CO2 as a pollutant in the same category as a truly toxic gas like carbon monoxide (CO). In fact CO2 is absolutely essential for plants and as a result for most life on this planet to exist. We would have much more to worry about if human activity was causing CO2 to decrease in the atmosphere, rather than causing it to increase. The issue of the government's authority to regulate CO2 emissions continues to be debated in the courts. In June 2014, the Supreme Court reaffirmed the pollutant status of CO2, but did set limits on the EPA's authority to regulate emissions, (see Supreme Court limits EPA's authority to regulate carbon dioxide emissions).
An opposing point of view was expressed to congress in Princeton University Physicist Dr. Will Happer's Statement before the US Senate's Environment and Public Works Full Committee Hearing on May 20, 2010. A paragraph from that testamony is quoted below.
"I keep hearing about the pollutant CO2, or about poisoning the atmosphere with CO2, or about minimizing our carbon footprint. This brings to mind another Orwellian pronouncement that is worth pondering: But if thought corrupts language, language can also corrupt thought. CO2 is not a pollutant and it is not a poison and we should not corrupt the English language by depriving pollutant and poison of their original meaning. Our exhaled breath contains about 4% CO2. That is 40,000 parts per million, or about 100 times the current atmospheric concentration. CO2 is absolutely essential for life on earth. Commercial greenhouse operators often use CO2 as a fertilizer to improve the health and growth rate of their plants. Plants, and our own primate ancestors evolved when the levels of atmospheric CO2 were about 1000 ppm, a level that we will probably not reach by burning fossil fuels, and far above our current level of about 389 ppm. We try to keep CO2 levels in our U.S. Navy submarines no higher than 8,000 parts per million, about 20 times the current atmospheric levels. Few adverse effects are observed at even higher levels."