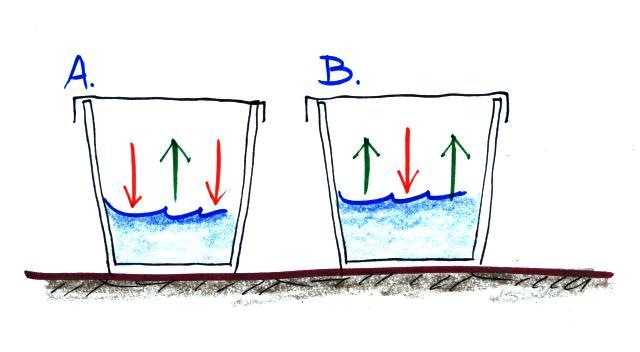
(i) The water in Glass A isn't
evaporating as quickly as the water in Glass B. The rate
of evaporation depends on water temperature. Warm water
(hot tea)
evaporates more rapidly than colder water (ice tea). The water in Glass A must be COLDER than in Glass B.
(ii) Water vapor in Glass A is condensing twice as rapidly as in Glass B. The rate of condensation depends on the amount of water vapor in the air. There must be
MORE water vapor in the air in Glass A than in Glass B to provide the higher rate of condensation.
When air is saturated (RH=100%) the rates of evaporation and condensation will be equal. This isn't the case in either glass above.
(iii) and (iv)
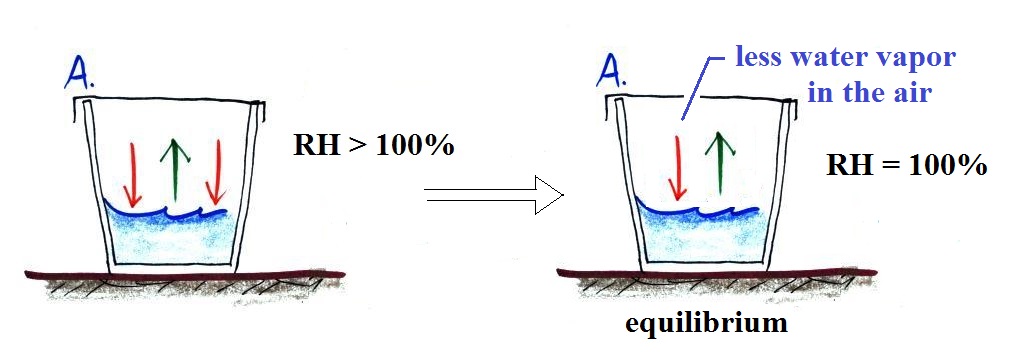
The air in Glass A is supersaturated (RH > 100%),
there's too much water vapor in the air in Glass A. This
is just a temporary imbalance. Water vapor is being
removed from the air by condensation at a faster rate than
water vapor is being added by evaporation. The amount of
water vapor in Glass A will decrease. As it decreases
the rate of condensation will drop and eventually become equal
to the rate of evaporation.
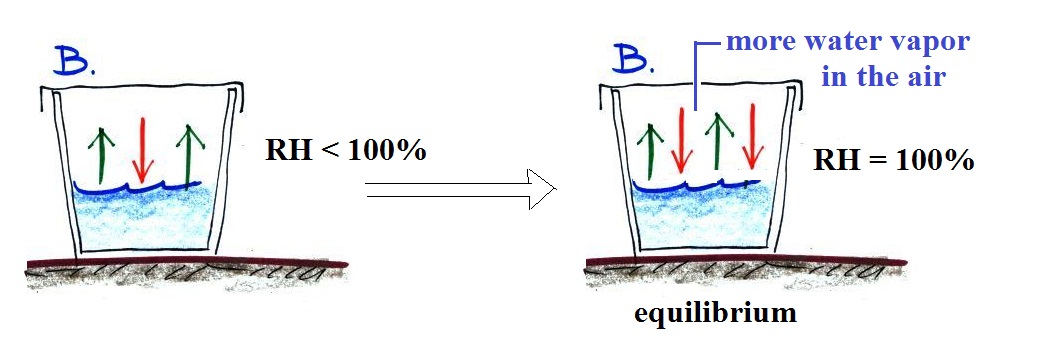
The RH of the air in Glass B is less than 100%. Because more moisture is being added to the air by evaporation than is being removed by condensation the amount of water vapor in the air in Glass B will increase. As it water vapor concentration builds the rate of condensation will increase. Eventually again the rate of condensation will become equal to the rate of evaporation. Equilibrium is achieved at that point.
evaporates more rapidly than colder water (ice tea). The water in Glass A must be COLDER than in Glass B.
(ii) Water vapor in Glass A is condensing twice as rapidly as in Glass B. The rate of condensation depends on the amount of water vapor in the air. There must be
MORE water vapor in the air in Glass A than in Glass B to provide the higher rate of condensation.
When air is saturated (RH=100%) the rates of evaporation and condensation will be equal. This isn't the case in either glass above.
(iii) and (iv)


The RH of the air in Glass B is less than 100%. Because more moisture is being added to the air by evaporation than is being removed by condensation the amount of water vapor in the air in Glass B will increase. As it water vapor concentration builds the rate of condensation will increase. Eventually again the rate of condensation will become equal to the rate of evaporation. Equilibrium is achieved at that point.