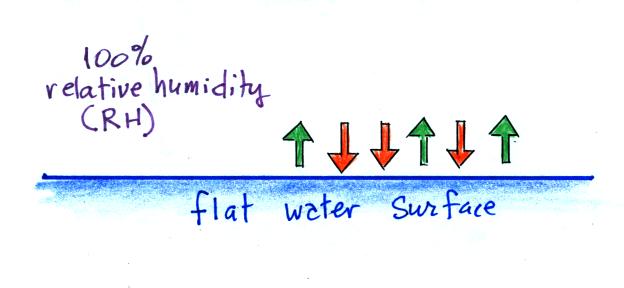
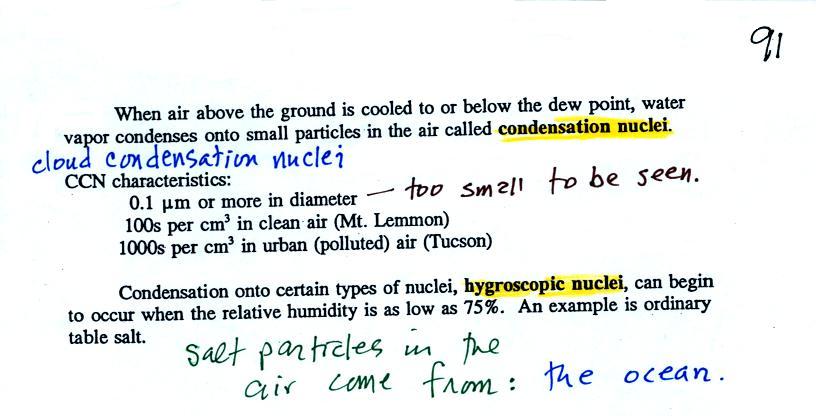
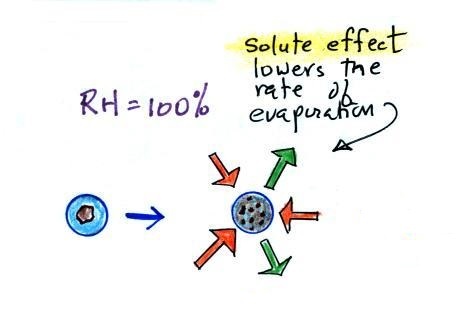
| it is much easier for water vapor to condense onto small particles called condensation nuclei |
rather than just
condensing and forming small droplets of pure water |

 |
 |
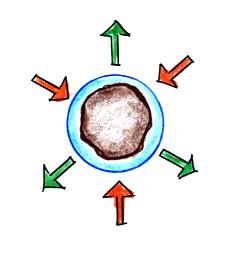
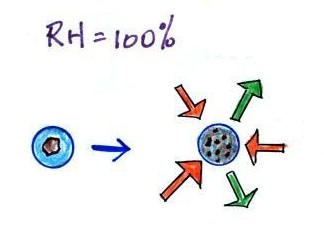
| solution droplet |
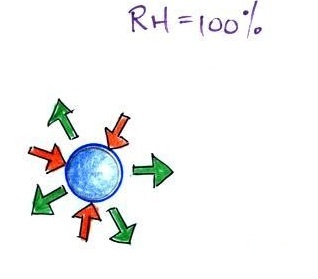
pure water droplet |
 |
 |
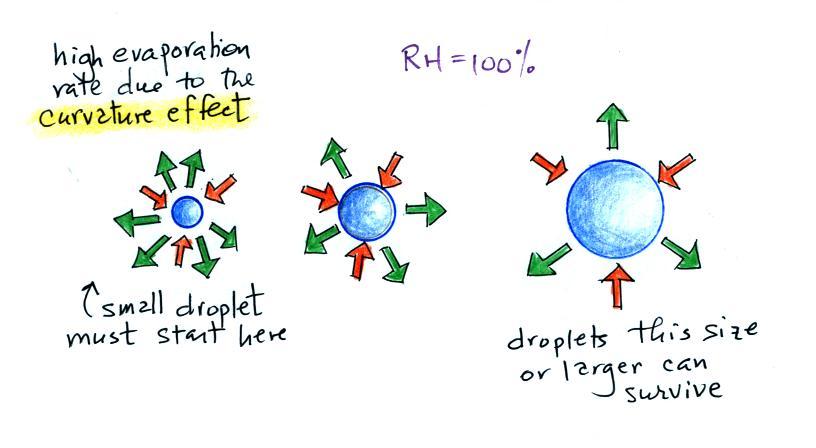
| the droplet is able to
grow |
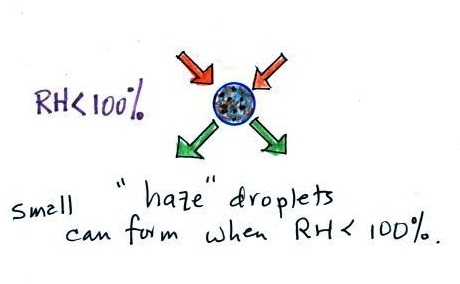
the droplet is in
equilibrium with its surroundings even when the RH is less than 100% |