Tuesday Sep. 30, 2016
Music from Lissie: "Record
Collector" (4:14) (recorded in the studios of KCMP FM,
Minnesota Public Radio), "In Sleep"
(5:23) part of a set at the end of the Guitar
Center's Singer/Songwriter 2 Competition Finals at Hotel
Café in Hollywood (Mar., 2013), "They All Want
You" (4:14), and "Further
Away" (4:14) from the Bing Lounge at 101.9 KINK FM
(Portland, OR), "Love
in the City" (3:46), and "The Habit"
Live at Hotel San Jose, SXSW 2013 (4:26).
Trace gases in air - pollutants and greenhouse gases
We are going to add a bunch of trace gases to the list of the
5 main gases in our present day atmosphere. Trace gases are
found in very low concentrations in air. The concentrations
often vary with location and time. The fact that the
concentrations are low doesn't mean the trace gases are not
important. This week we'll be concentrating on one sub-group
of trace gases, air pollutants. Air pollution (both indoors
and outdoors) probably kills a few million people every year
across the globe.
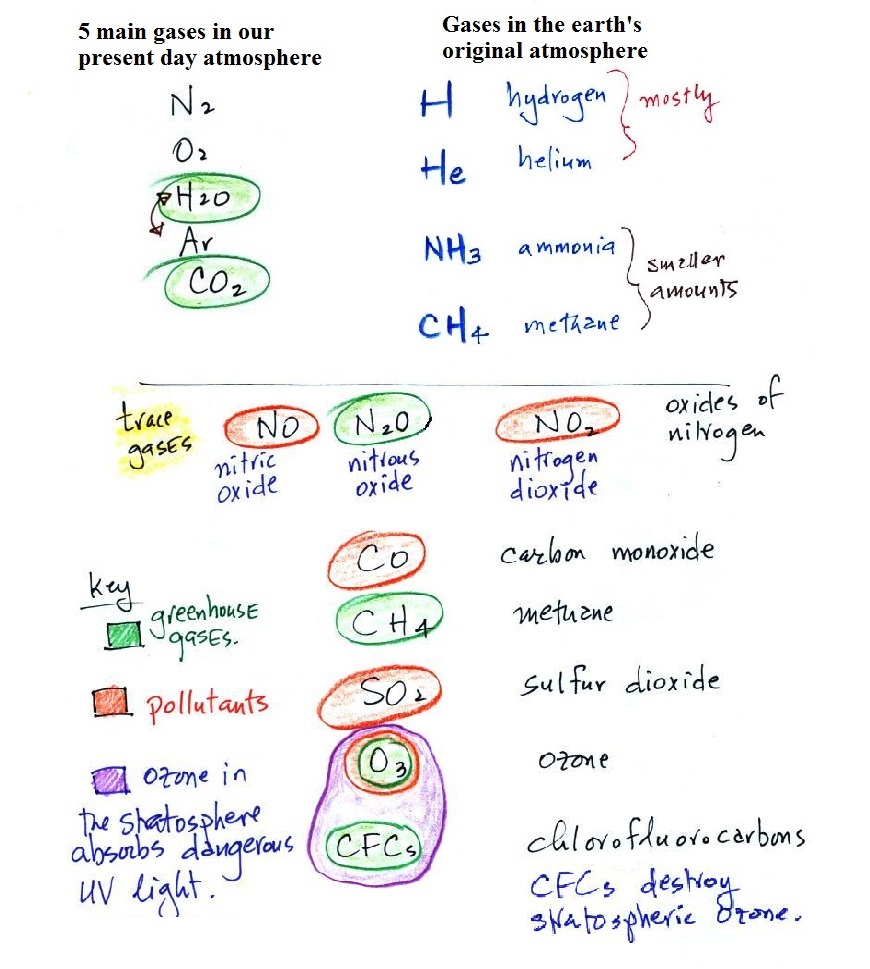
Water vapor, carbon
dioxide, methane, nitrous oxide
(N2O = laughing gas), chlorofluorocarbons, and ozone are all greenhouse
gases. The greenhouse effect warms the earth
and increasing atmospheric concentrations of these gases are
responsible for the current concern over climate change and
global warming. We'll discuss this topic and learn more
about how the greenhouse effect actually works later in the
course. (Carbon dioxide will probably be the subject of
a future 1S1P Assignment).
Carbon monoxide, nitric oxide, nitrogen
dioxide, ozone, and sulfur
dioxide are some of the major air pollutants.
We'll cover 3 of these in more detail this week.
I put Ozone in a group by itself. It has
sort of a Dr.
Jeckyl and Mr. Hyde personality
(i) Ozone in the
stratosphere (a layer of the atmosphere
between about 10 and 50 km altitude) is beneficial because it
absorbs dangerous (sometimes deadly) high energy ultraviolet
(UV) light coming from the sun. Without the protection of
this ozone layer, life as we know it would not exist on the
surface of the earth. It was only after ozone started to
buildup in the atmosphere that life could move from the oceans
onto land. Chlorofluorocarbons
are of concern in the atmosphere because they destroy
stratospheric ozone.
(ii) Ozone in the troposphere (the
bottom 10 kilometers or so of the atmosphere where we live)
ozone is a pollutant and is one of the main ingredients in
photochemical smog.
(iii)
Ozone is also a greenhouse
gas.
There's a good chance there won't be time to discuss the
photographs that follow, but they don't really require much
explanation.
Gases like water vapor, oxygen, and nitrogen are invisible.
Some gases are colored and can be seen; some examples are
shown below. I would like to bring some actual samples
to class, but most of these gases are toxic and require very
careful handling.
 |
|
 |
|
Bromine in both
liquid and gaseous phases. Bromine and mercury are the
only two elements that exist as liquids at room
temperature. The bromine is in a sealed glass
ampoule inside an acrylic cube. Bromine could be
safely brought to class in a container like this.
I actually have a sample of mercury in a cube like this
and will bring it to class during the semester.
Here's what Webelements.com says about bromine: " It is
a serious health hazard, and maximum safety precautions
should be taken when handling it." I'm not sure what
maximum safety precautions are, that's why I don't bring
it to class.
This photo was taken by Alchemist-hp and was Picture
of the Day on the English Wikipedia on Oct. 29, 2010.
|
Chlorine (Cl2)
I found this image here
|
Iodine
Also an element that is normally found in solid
form. The solid sublimes, i.e. it changes directly
from solid to gas (you would probably need to heat the
solid iodine to produce gas as deeply colored as seen in
the picture above). source
of this image
I think we can probably handle iodine safely and might
well bring some to class.
|
Nitrogen dioxide (NO2)
An important pollutant. I used to make this
in class but I've read that you can inhale a fatal
dose of NO2
before showing any symptoms. NO2
also has an anesthetic effect - it can deadens your
sense of smell.
source
of this image
|
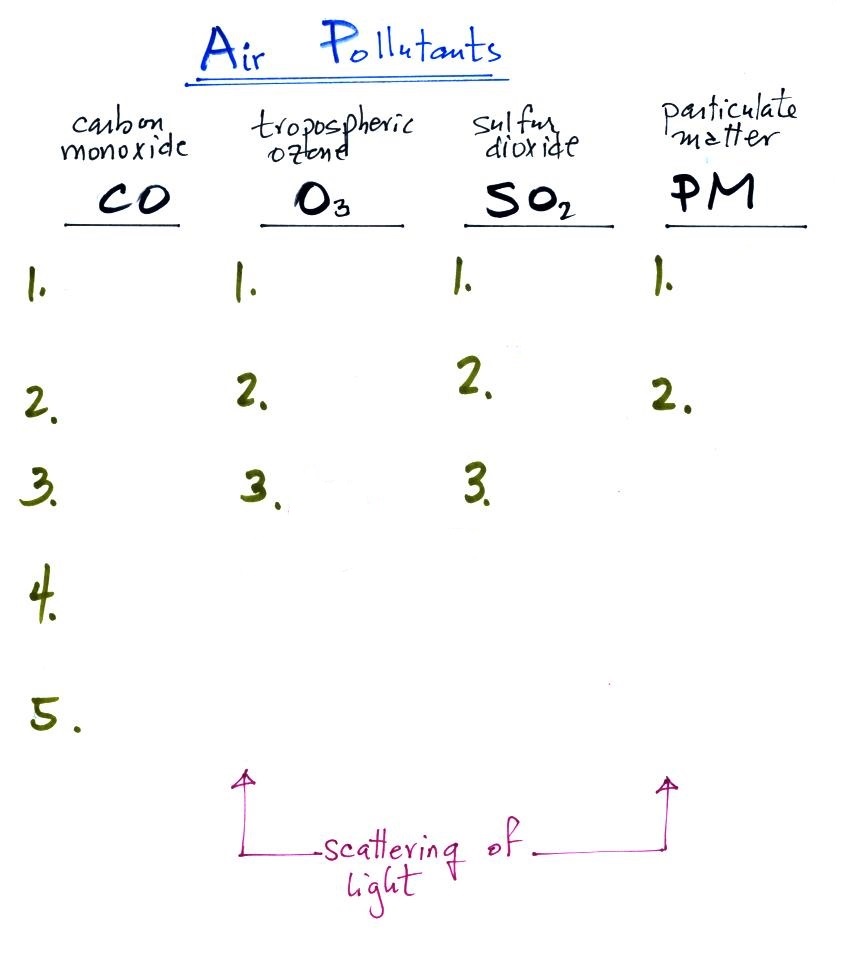
Air Pollutants
For much of this week we will begin looking at four
air pollutants. They are carbon monoxide, tropospheric
ozone, sulfur dioxide (all of these are gases). We'll also
cover particulate matter. They're listed below together with
an idea of the number of main points you should remember and
understand about each.
Today's class will also feature a light
scattering demonstration. It's a fairly simple concept and
explains how/why we are able to see things like smog, clouds,
and particulate matter in the air. We will also produce
some photochemical smog in a demonstration Thursday probably
(safely confined in a glass bottle). You'll be able to see
the smog because it scatters light.
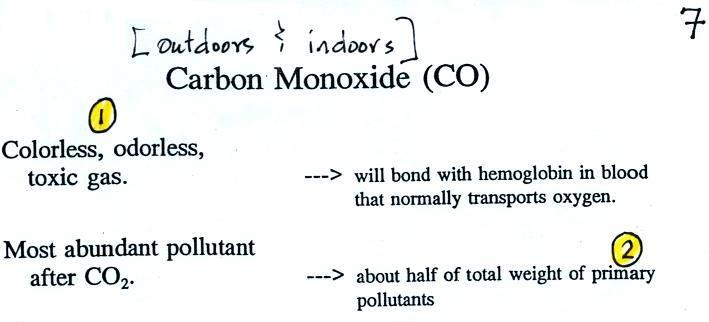
Carbon Monoxide (CO)
We'll start our section on air
pollutants with carbon monoxide. You'll find
additional information on carbon monoxide and other air
pollutants at the Pima
County Department of Environmental Quality website
and also at the US
Environmental Protection Agency website.
The material above is from page 7 in the photocopied
ClassNotes. We will mostly be talking about carbon
monoxide found outdoors, where it would only rarely reach fatal
concentrations. CO is a serious hazard indoors also where
it can (and does) build up to deadly concentrations (several
people
were
almost
killed
in
Tucson
in
December 2010 for example).
Between
1999 and 2010 an average of 430 people were killed per
year from unintentional, non-fire-related carbon monoxide
poisoning according to the Centers for Disease Control and
Prevention (ref).
Carbon monoxide is insidious, you can't smell it or see it
and it can kill you (Point 1).
Once
inhaled,
carbon
monoxide
molecules
bond
strongly
to
the
hemoglobin
molecules
in
blood
and
interferes
with
the
transport
of
oxygen
throughout
your
body.
The
article above about carbon monoxide poisoning in Tucson mentions
that the victims were put inside a hyperbaric (high pressure)
chamber filled with pure oxygen. This must force oxygen
into the blood and displace the carbon monoxide molecules.
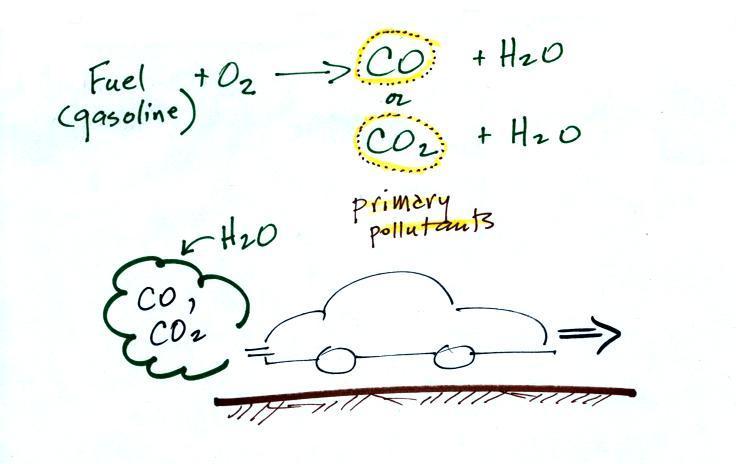
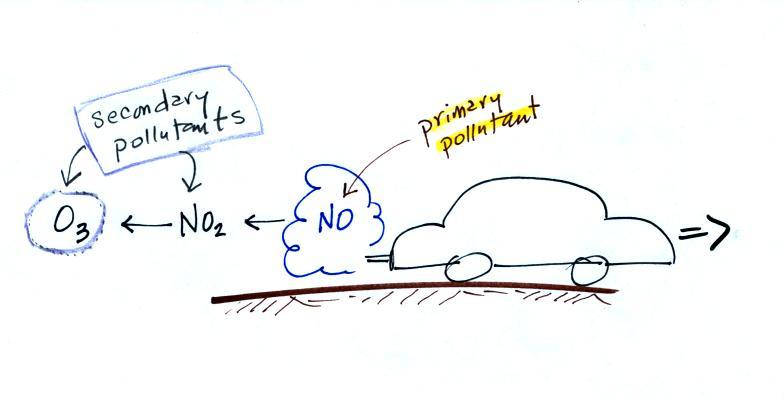
CO is a primary pollutant (Point
2 above). That means it goes directly from a
source into the air, CO is emitted directly
from an automobile tailpipe into the atmosphere for example.
The difference between primary and secondary pollutants
is probably explained best in a series of pictures.
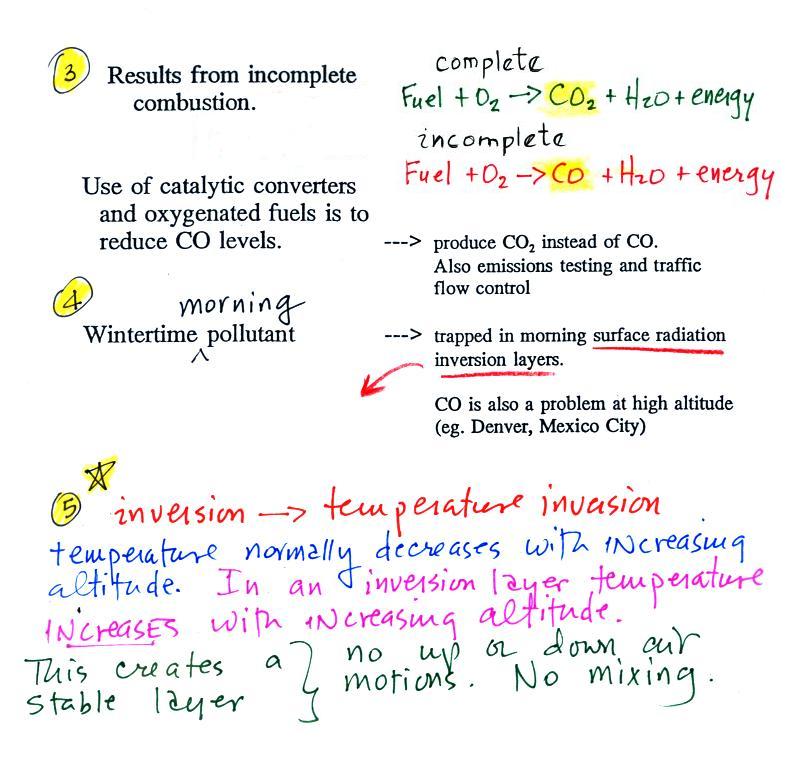
Point 3 explains that CO is produced by incomplete
combustion of fossil fuel. Basically there isn't enough
oxygen. More oxygen and complete combustion would produce
carbon dioxide, CO2.
Because cars and trucks produce much of the
CO in the atmosphere in Tucson, special
formulations of gasoline (oxygenated fuels) are used during the
winter months in Tucson to try to reduce CO emissions. The
added ethanol has the effect of adding more oxygen to the
combustion process.
Vehicles are also fitted with a catalytic converter that
will change CO into CO2 (and
also NO into N2 and O2 and hydrocarbons into H2O and CO2).
In Pima County, vehicles must also pass an emissions test every
year to insure that the car is burning fuel as cleanly as
possible.
The photograph below at left shows the blue flame that results
from complete combustion of natural gas (methane) in a bunsen
burner. The air holes on the side of the bunsen burner are
open and plenty of air (oxygen) is available during
combustion. The flames on a gas stove or the pilot light in
a hot water heat or a furnace should have this blue color.
If the air holes are closed, the flame turns yellow. There
isn't enough oxygen in this case. This incomplete combustion
will produce more carbon monoxide and also a lot of black soot
(carbon). (source
of the photo)
Clean (complete) and dirty (incomplete)
combustion of natural gas
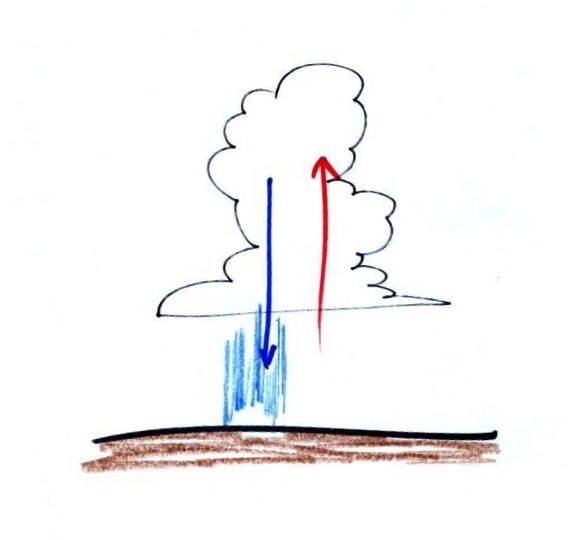
In the atmosphere CO concentrations peak on winter mornings (Point 4). The reason for
this is surface radiation inversion layers. They are most
likely to form on cold winter mornings.
When we say inversion layer (Point 5), we mean a temperature inversion, a
situation where air temperature increases with increasing
altitude. That's just the opposite of what we are used to
(you would expect it to be colder at the summit of Mt. Lemmon than
here in the Tucson valley). This produces stable atmospheric
conditions which means there is little up or down air motion.
The lack of vertical air motions means there is very
little vertical mixing in a stable air layer.
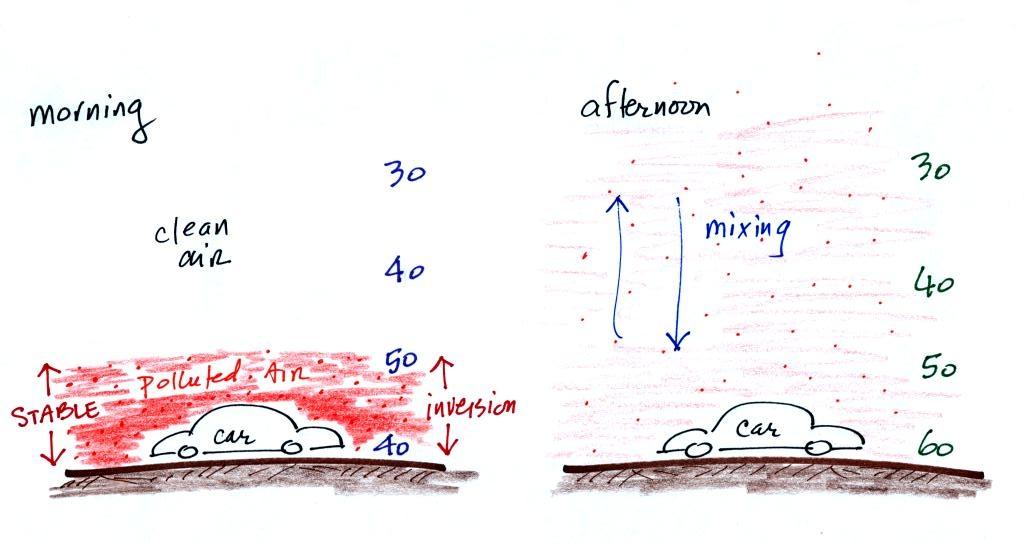
In the left figure above, notice how temperature increases
from 40 F to 50 F in the thin air layer next to the ground.
That's the inversion layer. Temperature then begins to
decrease as you move further up. When CO is emitted into the
thin stable layer during the morning rush hour, the CO remains in
the layer and doesn't mix with cleaner air above. CO
concentrations build. Later in the day the
ground and air in contact with the ground warms. The
inversion disappears and air at the ground mixes with cleaner air
above. The evening rush hour adds CO to the air but it is
mixed in a larger volume of air and the concentration doesn't get
as high.
Thunderstorms like you have been seeing this time of year
contain strong up and down air motions. Thunderstorms are an
indication of unstable atmospheric conditions.
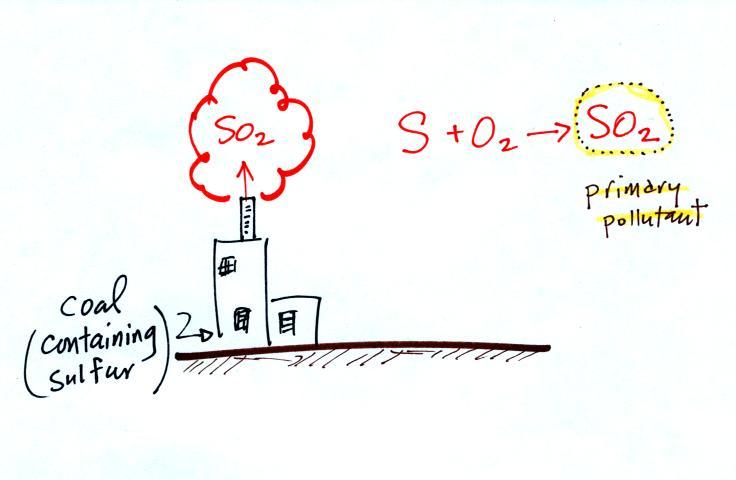
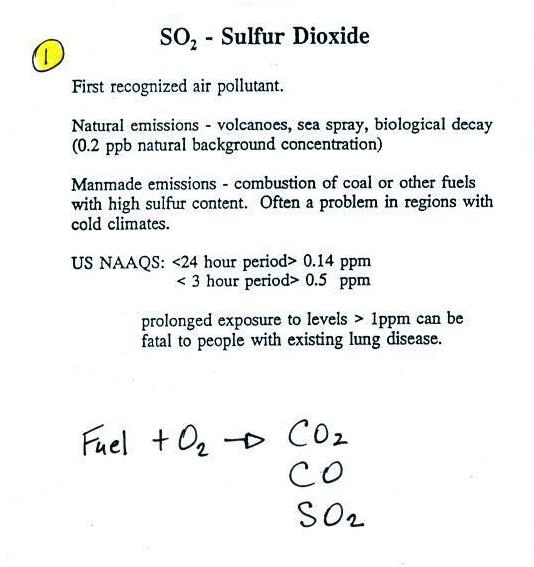
Sulfur dioxide (SO2
)
We'll turn now to another of the air
pollutants we will cover, sulfur
dioxide (SO2 ).
Sulfur dioxide is produced by the combustion of sulfur
containing fuels such as coal. Combustion of fuel also
produces carbon dioxide and carbon monoxide. People probably
first became aware of sulfur dioxide because it has an unpleasant
smell. Carbon dioxide and carbon monoxide are
odorless. That is most likely why sulfur dioxide was the
first pollutant people became aware of.
I've checked on the smell of sulfur dioxide class and have found
two descriptions: one described it as the smell of rotten eggs (I
associate that with hydrogen
sulfide, H2S, which is also
poisonous), the second is a pungent irritating odor which is what
I remember. Apparently sulfur dioxide is one of the smells
in a freshly struck match.
Volcanoes are a natural source of sulfur dioxide.
London-type smog

The inversion layer in this case lasted for several days and was
produced in a different way than the surface radiation
inversions we heard about when covering carbon monoxide.
Surface radiation inversions usually only last for a few hours.
The term smog, a contraction of smoke + fog, was invented to
describe a mixture of smoke and fog, something that was fairly
common in the winter in London. The 1952 event was an
extreme case. Now we distinguish between "London-type
smog" which contains sulfur dioxide and photochemical or "Los
Angeles-type smog" which contains ozone.
Most of the photographs below come
from articles published in 2002 and 2012, the
50th and 60th anniversaries of the event.
The caption to this
photo from The Guardian reads
"Arsenal goalkeeper Jack Kelsey peers into the
fog.
The 'smog' was so thick the game was eventually
stopped."
|

The smog in this photo is the thickest I was able to
find. Visibility here is perhaps 10 or 20 feet.
(source
of this image)
|
|
|
|
Smog masks from this
reference
The masks would filter out the smoke but not the
sulfur dioxide gas
|
Here are some interesting photographs
of early and mid 20th century London.
The sulfur dioxide didn't
kill people directly. Rather it
would aggravate an existing condition of some
kind. The SO2 probably also
made people susceptible to bacterial infections
such as pneumonia. Here's
a link that discusses the event
and its health effects in more detail.
The Clean
Air Act of 1956 in England reduced smoke
pollution and emissions of sulfur dioxide.
Air
pollution disasters involving sulfur dioxide have also
occurred in the US. One of the deadliest events was in
1948 in Donora, Pennsylvania.
The reference
material that contained this photographed clearly
stated "This eerie photograph was taken at noon on
Oct. 29, 1948 in Donora, PA as deadly smog enveloped the
town. 20 people were asphyxiated and more than 7,000
became seriously ill during this horrible event."
The photograph below shows some of the mills that were operating
in Donora at the time. Not only where the factories adding
pollutants to the air they were undoubtedly adding hazardous
chemicals to the water in the nearby river.
source
of this photo
The US passed its own
Clean Air Act in 1963. There have been several
major revisions since then. The EPA began
in late 1970 (following an executive order signed by President
Nixon)
"When Smoke Ran Like Water,"
a book about air pollution is among the books
that you can check out, read, and report on to
fulfill part of the writing requirements in this
class (though I would encourage you to do an
experiment instead). The author, Devra
Davis, lived in Donora Pennsylvania at the time
of the 1948 air pollution episode.
Scattering (splattering) of light
We spent the next portion of today's class learning about the
scattering of light. You are able to see a lot of
things in the atmosphere (clouds, fog, haze, even the blue sky)
because of scattering of light. We'll try to make a cloud of
smog in class on Friday. The individual droplets making up
the smog cloud are too small to be seen by the naked eye.
But you will be able to see that they're there because the
droplets scatter light. That's true also of the little water
droplets that make up a cloud. So we need to take some time
for a demonstration to see exactly what light scattering is.
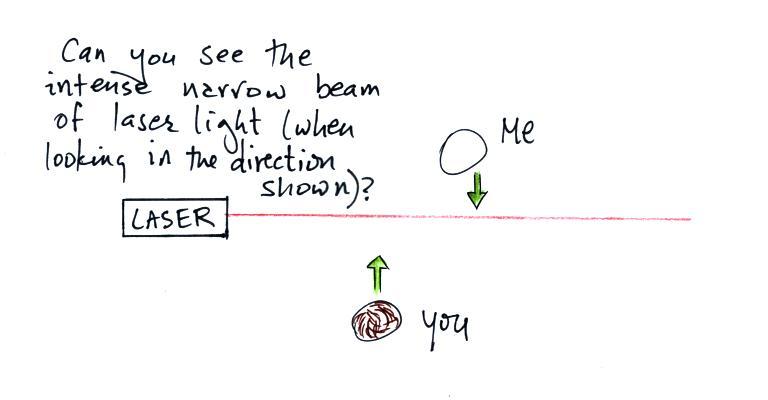
In the first part of the demonstration a narrow beam of intense
red laser light was directed from one side of the classroom to the
other.
We're looking down from above in the the figure
above. Neither the students or the instructor could see
the beam of light. To see the laser light some of it
would need to be traveling toward you rather than from one
side of the room to the other. This figure
and the ones that follow are on pps 107a & 107b on the
packet of ClassNotes.
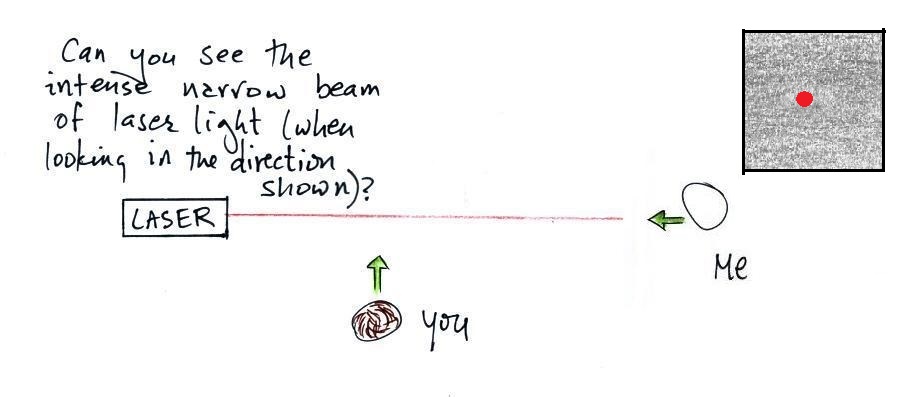
The instructor would have been able to see
the beam if he had stood at the end of the beam of laser light
where it hit the wall and looked back along the beam of light
toward the laser. The insert at upper right shows what
the instructor would see, a bright spot of light originating
at the end of the laser tube itself. That wouldn't have
been a smart thing to do, though, because the beam was strong
enough to possibly damage his eyes (there's a
warning on the side of the laser).
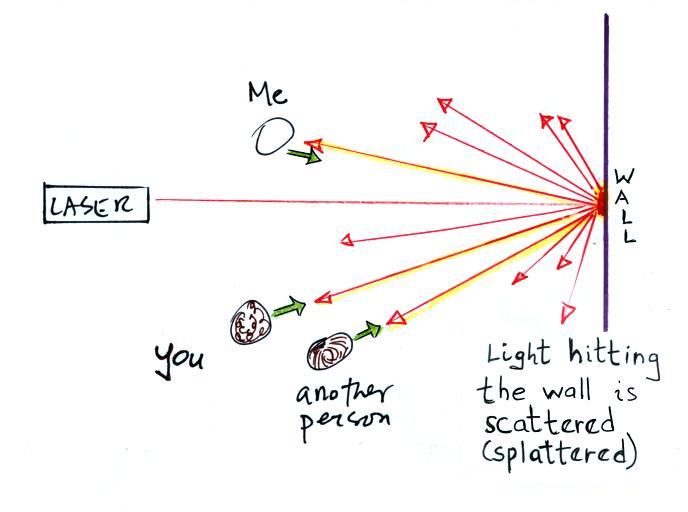
Everybody was able to see a bright red spot where the laser
beam struck the wall.
This is because when the intense beam
of laser light hits the wall it is scattered (I think
splattered is a more descriptive term). The
original beam is broken up into a multitude of weaker
rays of light that are sent out in all directions.
There is a ray of light sent in the direction of every
student in the class. They see the red spot of
light because they are looking back in the direction the
ray came from. It is safe to look at this
light because the original intense beam is split up into
many much weaker beams.
Next we clapped two erasers together so that some
small particles of chalk dust fell into the laser
beam. I also sprinkled baby powder into the beam.
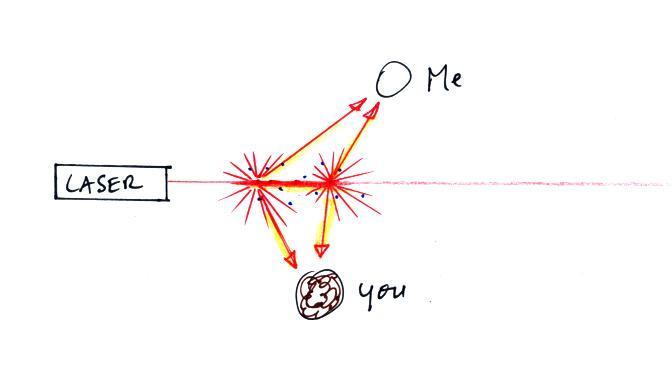
Now instead of a single spot on the wall, students saws lots of
points of light coming from different positions along a straight
segment of the laser beam. Each of these points of light was
a particle of chalk, and each piece of chalk dust was intercepting
laser light and sending light out in all directions. Each
student saw a ray of light coming from each of the chalk
particles. With a cloud of chalk dust you are able to see
segments of the laser beam.
We use chalk because it is white, it will scatter rather than
absorb visible light. What would you have seen if black
particles of soot had been dropped into the laser beam?
In the last part of the demonstration we made a cloud by pouring
some liquid nitrogen into a cup of water. The cloud droplets
are much smaller than the chalk particles but are much more
numerous. They make very good scatterers.
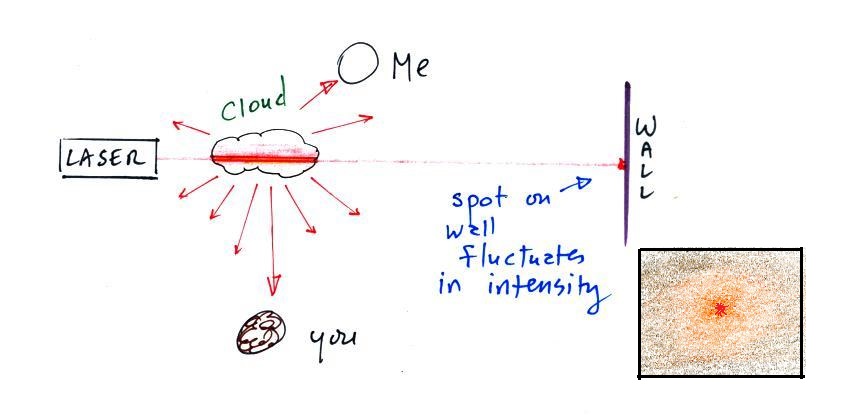
The beam of laser light was very bright as
it passed through the small patches of cloud. The cloud
droplets did a very good job of scattering laser light.
So much light was scattered that the spot on the wall
fluctuated in intensity (the spot dimmed when lots of light
was being scattered, and brightened when not as much light was
scattered). Again the insert shows what you would see if
you stood at the wall and looked back toward the laser.
Some of the light passes through the cloud so you would still
be a spot of red light, but it would be weaker and more
diffuse. Then you would see red scattered light coming
from the cloud surrounding the beam of laser light.
Here's a side view photo that I took back in my office.
The laser beam is visible in the left 2/3 rds of the picture
because it is passing through cloud and light is being scattered
toward the camera. There wasn't any cloud on the right
1/3rd of the picture so you can't see the laser beam over near
Point 1.
The air molecules in the room are actually scattering laser
light but it's much too weak for us to be able to see it.
When a stronger light source (sunlight) shines through much more
air (the entire atmosphere) we are able to see the scattered
light. The blue light that you see when you look at sky is
sunlight being scattered by air molecules. This will be
the topic of another, soon to appear, 1S1P Assignment.