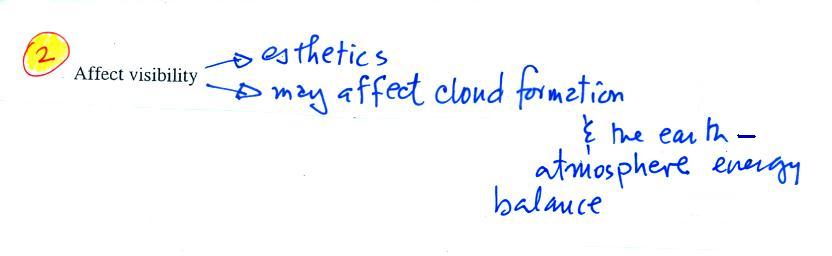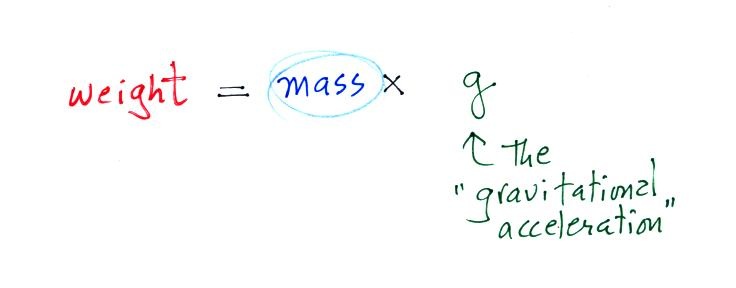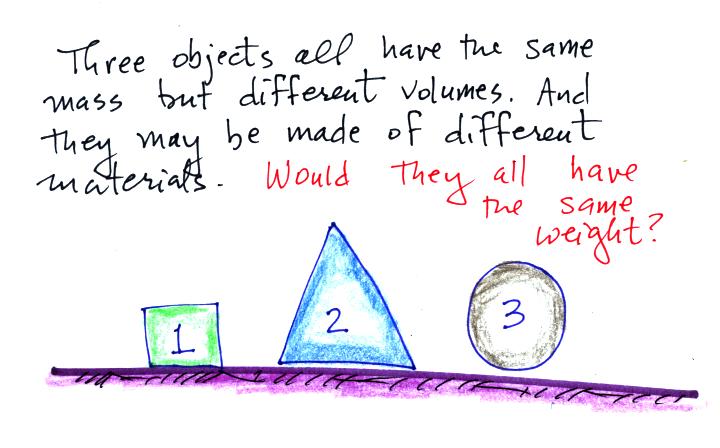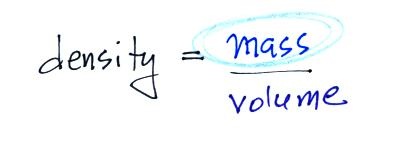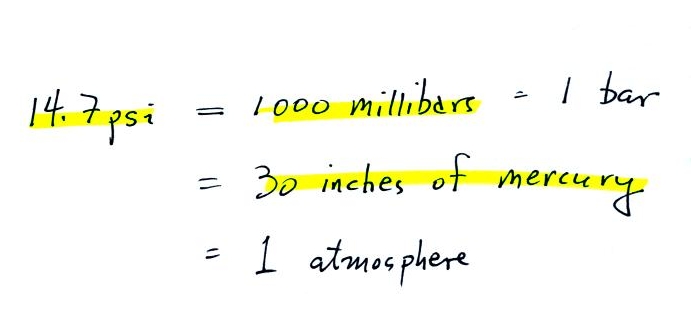Thursday Sep. 3, 2015
Music this morning from Joss Stone "Love Me"
& "Molly
Town" and Elle King "Good for
Nothin Woman" & "Under the
Influence"
The Practice Quiz is one week from today (Thu., Sept.
10). I will try to get a study guide online one week before
each of this semester's quizzes. So here is a preliminary
version of the Practice Quiz Study Guide.
There may be some small changes made by early next week, because
it's not clear at this point whether we will be able to get
through the last topic or two on the study guide by the end of
class next Tuesday. There will be reviews Tuesday and
Wednesday afternoon next week even though this is just a practice
quiz. Locations of the review sessions aren't yet known.
The first of the 1S1P
Assignment #1 reports is due next Tuesday.
Acid Rain Demonstration
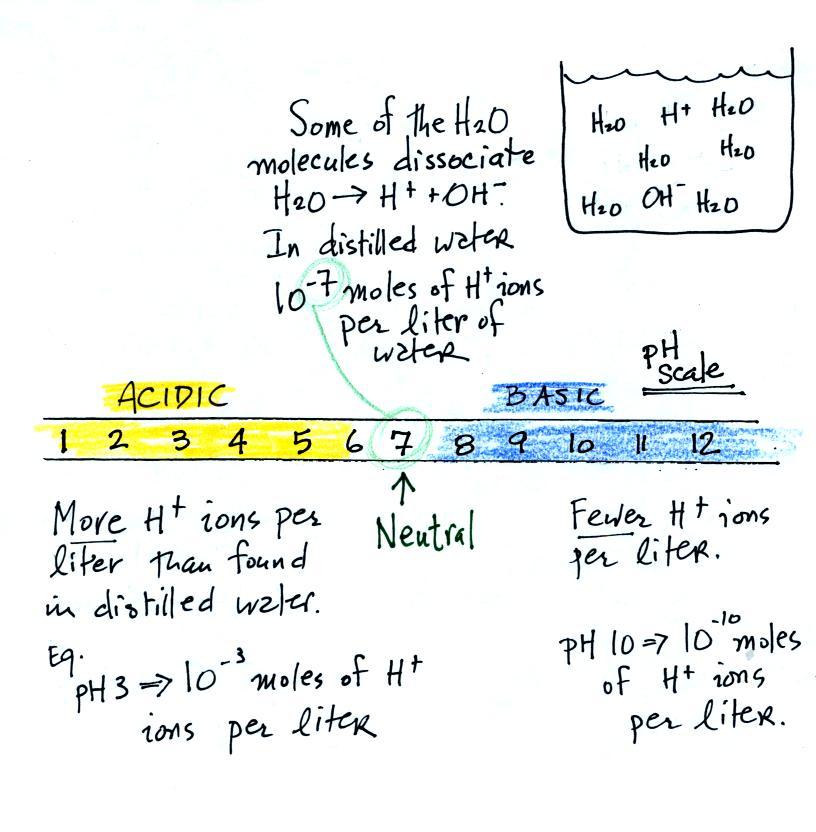
Some common acids are listed below. In
solution the acid molecules dissociate (split) into pieces.
The presence of H+ ions is what makes these materials
acids.
Actually
it isn't enough to just have H+ ions for something to
be an acid, the H+ ion concentration must
be greater than is found in distilled water. Distilled water
is neither acidic nor basic, it's neutral. The H+
ion concentration in distilled water is 10-7 moles
of H+ ions per liter of
water. A mole is
just a number, a very large number (6 x 1023).
It's the same idea as dozen. A dozen means you've got 12 of
something. 10-7 moles
per liter is 6 x 1016 H+
ions per liter of water.
We often use the pH scale to measure acid concentration. An
H+ ion concentration of 10-7 moles/liter corresponds to
pH 7 (the pH value is computed by taking the -log10
of the H+
ion concentration). Other than remembering the pH
value of distilled water is pH7, these are all details
you don't need to worry about.
A basic solution will have an H+
ion concentration that is lower than found in pure water.
Pouring some acid into water would increase the H+ ion concentration (from 10-7moles/liter to 10-3moles/liter, perhaps as shown
in the example above). Adding a base to water will decrease
the H+ ion
concentration (from 10-7moles/liter
to 10-10moles/liter,
perhaps).
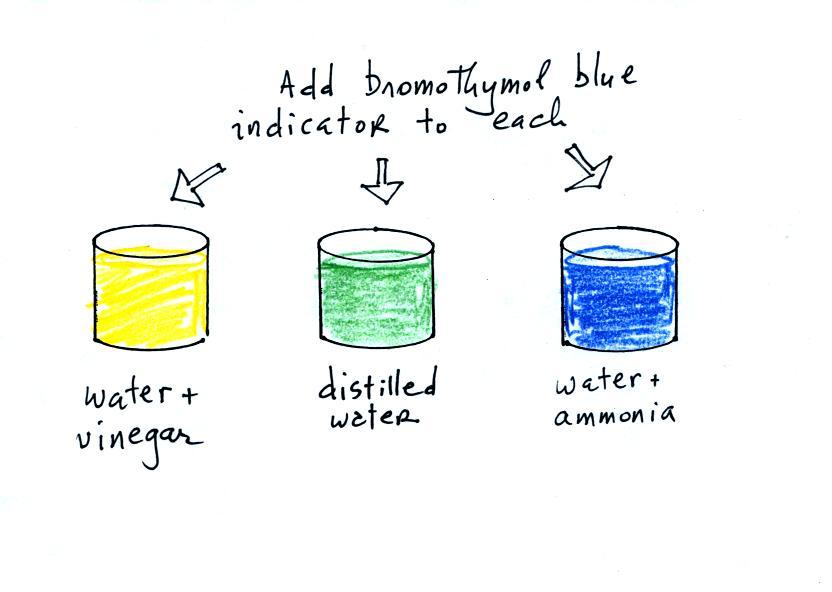
Now we can proceed with the demonstration. We will start
with three 1000 mL beaker each filled with distilled water.
Some vinegar (contains acetic acid) was added to the left beaker.
Some ammonia (a base) was added to the right beaker.
Then we added some bromothymol blue, a color indicator
solution, to all three beakers. Bromothymol blue has
the amazing property of changing color depending on whether it is
mixed with an acid (yellow or orange) or a base (deep blue).
So far
we just reviewed the pH scale and introduced acid/base indicator
solutions.
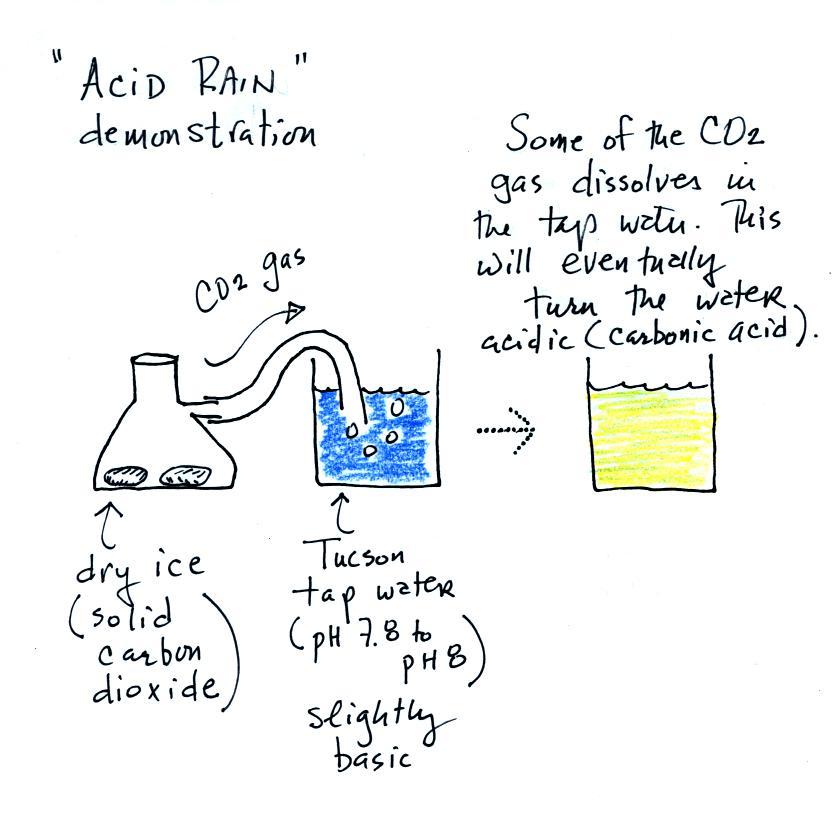
When sulfur dioxide is released into the air it reacts with the
water in clouds to produce acid rain. I really can't use SO2
in class because it's poisonous. I'll use carbon dioxide, CO2,
instead.
We added some Tucson tap water to a large 2000 mL beaker.
This represents a cloud. Tucson tap water is slightly
basic. We know that because it turned blue when we added
bromothymol blue to it. A few small pieces of dry ice are
put into a flask. We close the flask with a stopper.
The end of a piece of tubing connected to the flask is immersed in
the tap water.
Dry ice sublimates. It turns directly from solid to ice
(ordinary ice melts and turns from solid to liquid). The
gaseous CO2 is invisible but
you can tell it is there because of the bubbles in the tap
water. Some of the CO2
dissolves as it bubbles through the water and slowly turns the
water acidic. You can tell that this is occurring because
the bromothymol blue indicator turns from deep blue to green and
eventually to yellow.
I call this a "sort of" acid rain demonstration. That's
because we haven't really produced acid rain. Air contains
carbon dioxide and the CO2 makes natural rain slightly acidic (pH5.6 or
so). To make true acid rain we would need a different gas,
something other than carbon dioxide, something that would lower
the pH below 5.6.
While we didn't actually produce acid rain, there is concern
that increasing atmospheric concentrations of carbon dioxide will
dissolve and acidify the world's oceans. This is discussed
in the following article from The Christian Science Monitor.
You can download a copy of the article here.
The main concern over increasing atmospheric carbon dioxide
concentrations is global warming from enhancement of the
greenhouse effect. We will discuss this topic at some point
during the semester.
Carbonated beverages contain dissolved carbon dioxide and are
acidic. Soft drinks also contain phosphoric acid which makes
them even more acidic than the dissolved carbon dioxide would
do. With time the acidity of soft drinks can damage tooth
enamel.
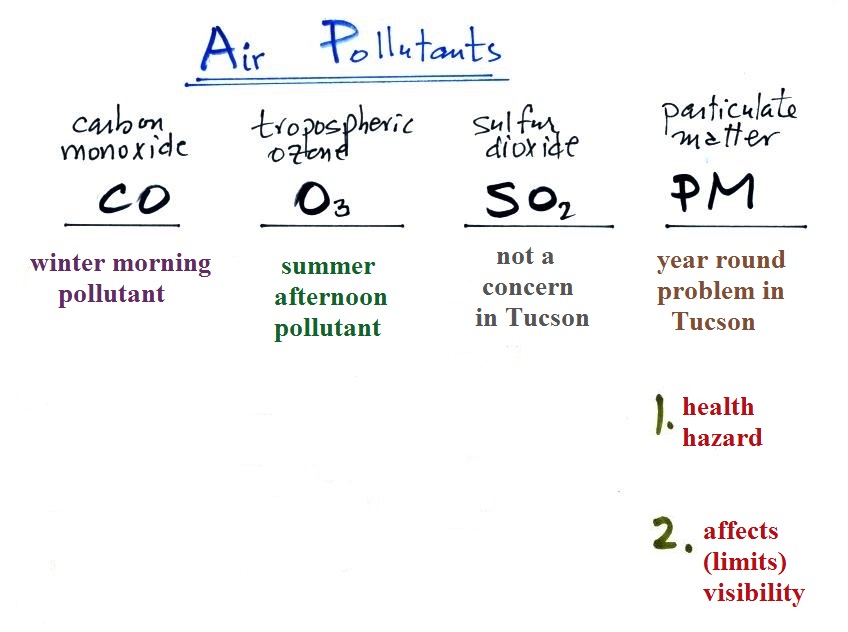
Particulate matter (PM)
The last pollutant that we will cover is Particulate Matter
(PM). This is small solid particles or drops of liquid, not
gases, that remain suspended in the air.
Carbon monoxide (CO), O3 , and
Particulate Matter are the three main pollutants of concern in
Tucson. PM is a year round problem in Tucson.
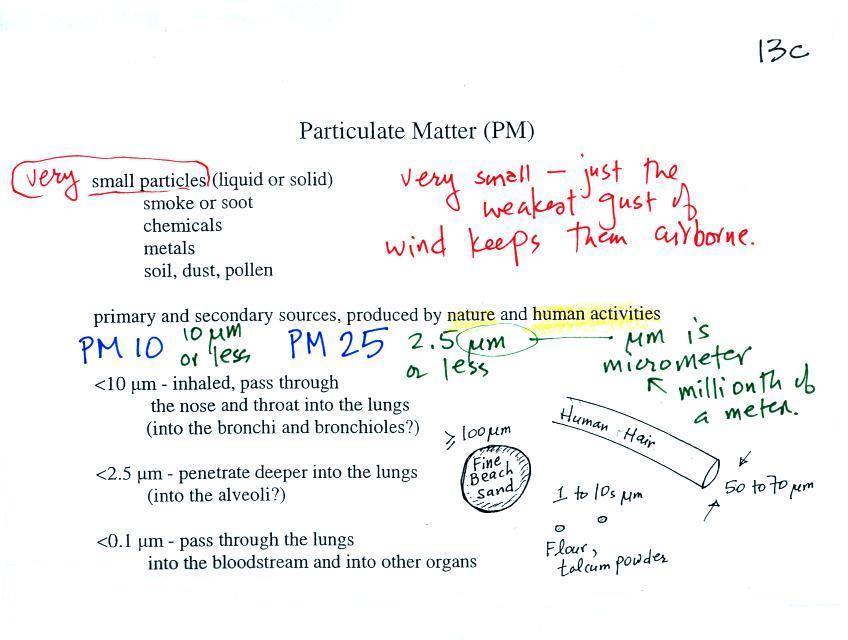
PM pollution is often split into two groups: PM10 and PM2.5.
These refer to particles with diameters less than 10 micrometers
and 2.5 micrometers, respectively. A micrometer (µm)
is one millionth of a meter (10-6
m). You'll find examples of metric distances ranging
from kilometers to nanometers at this
interesting site.
Particulate matter can be
produced naturally (wind blown dust, clouds above volcanic
eruptions, smoke from lightning-caused forest and brush
fires). Human activities also produce particulates
(automobile exhaust for example). Gases sometimes react in
the atmosphere to make small drops or particles (this is what
happened in the photochemical smog demonstration). Just
the smallest, weakest gust of wind is enough to keep these small
particles suspended in the atmosphere.
Sizes (in µm) of some common items are
sketched above. Better than sketches are some actual
photographs. I went looking for some yesterday. The
particles are so small they need to be examined using a
microscope.
Electron microscope
photograph of human red blood cells..
Individual cells in this example are a little
over 5 um in diameter.
This first
example is not something you'd find in the atmosphere.
(image source: Dartmouth College
Electron Microscope Facility)
This is something that is commonly found in the air.
This is a photograph of a mixture of different types of
pollen.
The largest pollen grain comes from morning glory (I think) and
is about 100 um in diameter
(image source: Dartmouth
College Electron Microscope Facility)

Scanning electron microscope photograph of volcanic ash
(USGS image by A.M. Sarna-Wojcick from
this source)
Airborne particulate matter
collected on the surface of a tree leaf (source).
These particles are pretty small with diameters of 1
to 2 µm.
According to the source, trees capture
appreciable amounts of particulate matter and remove it
from the air in urban areas.
One of the main concerns with particulate pollution is that the
small particles might be a health hazard ( a health advisory is
sometimes issued during windy and dusty conditions in Tucson)
Particles with dimensions of 10 µm
and less can be inhaled into the lungs (larger particles get
caught in the nasal passages). These inhaled particles
may be poisonous, might cause cancer, damage lung tissue, or
aggravate existing respiratory diseases. The smallest
particles can pass through the lungs and get into the blood
stream (just as oxygen does) and damage other organs in the
body.
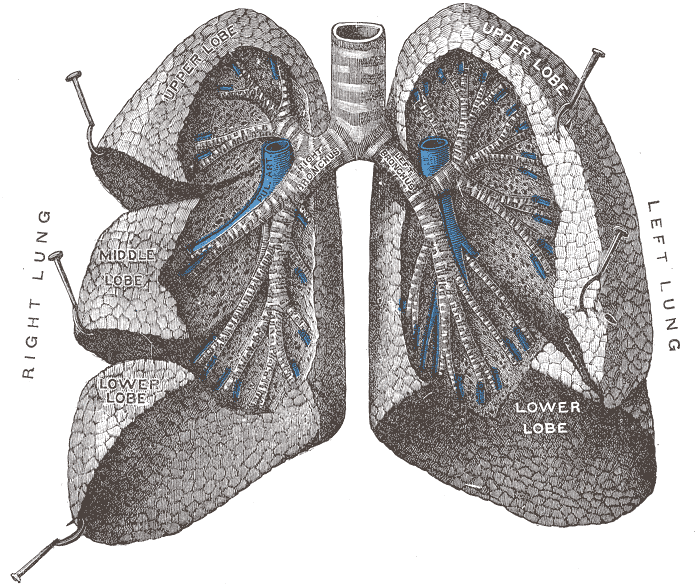
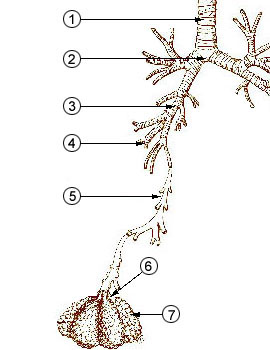
The figure below identifies some of the parts of the human
lung mentioned above. The key point is that
the passageways get smaller and smaller the deeper you move
into the lungs. The smallest particles are the most
dangerous because they can penetrate furthest into the lungs.
This next
portion of material wasn't covered in class.
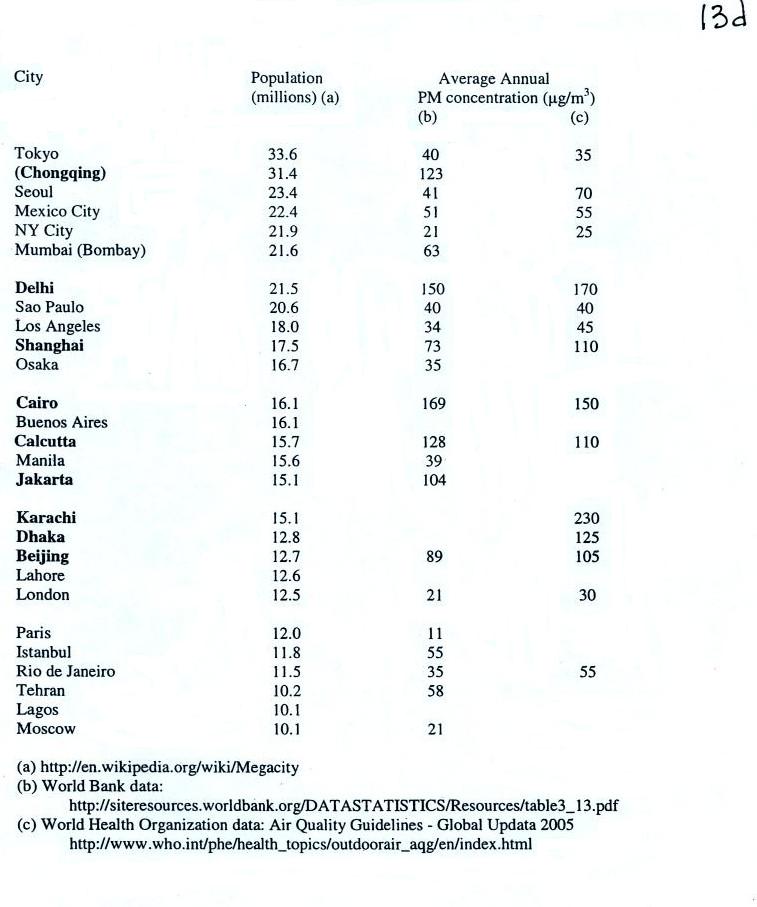
Note
the PM10 annual National Ambient Air Quality Standard
(NAAQS) value of 50 micrograms/cubic meter (µg/m3)
at the bottom of p. 13c in the photocopied
ClassNotes.
The following list (p. 13d in the ClassNotes) shows that there
are several cities (in bold font) around the world where
PM concentrations are 2 or 3 times higher than the NAAQS value.
Back to material that was mentioned in
class.
The 2008 Summer Olympics were held in Beijing and there
was some concern that the polluted air would affect the
athletes performance. Chinese authorities restricted
transportation and industrial activities before and during
the games in an attempt to reduce pollutant
concentrations. Rainy weather during the games may
have done the greatest amount of good.
Clouds and precipitation are the best way of cleaning
pollutants from the air. We'll learn later in the semester
that cloud droplets form on small particles in the air called
condensation nuclei. The cloud droplets then form raindrops
and fall to the ground carrying the particles with them.
The second main concern with particulates is the
effect they may have on visibility (esthetics below should
actually be spelled aesthetics - i.e. qualities that might
make something appear beautiful or not).
Here's a view of the Catalina mountains taken from the Gould
Simpson Building on the south side of campus.
Some rainy weather had occurred just a day to two earlier, cleaned
the air, and the visibility was very good. Clouds and rain
have done a really good job of cleaning the air.
Windy weather a few days later
stirred up a lot of dust that was carried into town.
This picture was taken the day after the windy weather.
There is still a lot of fine dust particles in the air and the
visibility is pretty bad.
We looked at some photographs from Beijing
(January, 2013) last week. Here are some pictures from Harbin,
China (October, 2013). That's about as bad as visibility can
get, visibility in some cases is just a few 10s of feet.
Also a picture from Paris
(March, 2014) (I might not have shown
that in class).
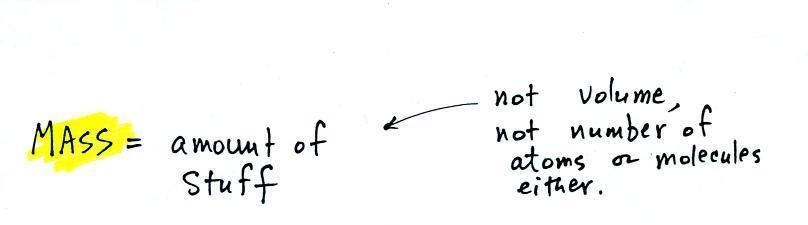
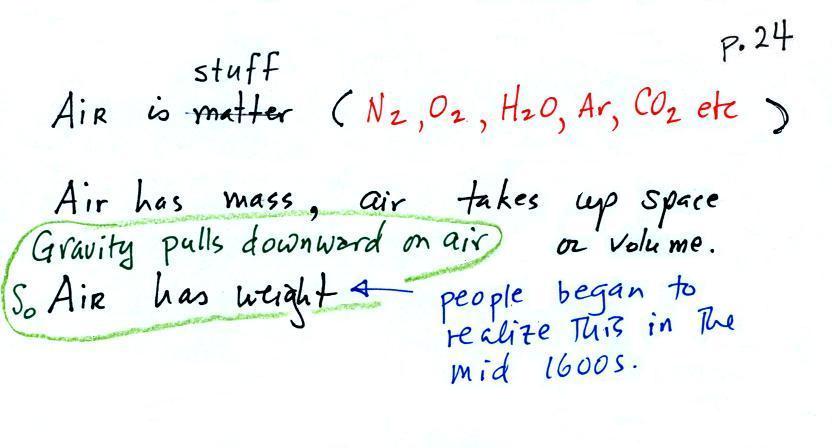
Mass,
weight, density, and pressure.
We spent the remainder of the day and
will spend next Tuesday on this topic. The classroom presentation
was probably pretty confusing.
Please read through this material on your
own.
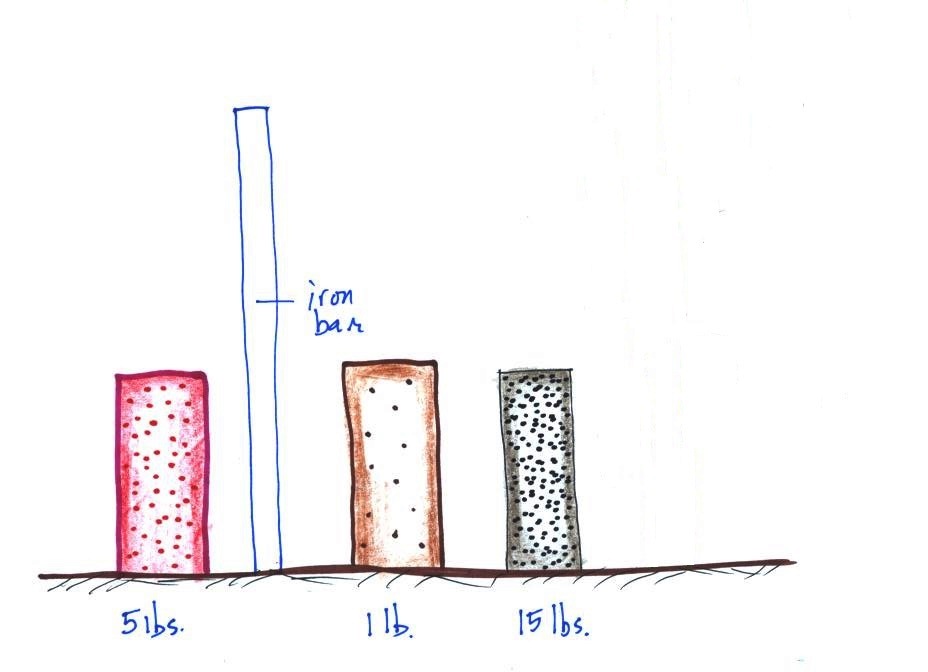
Weight
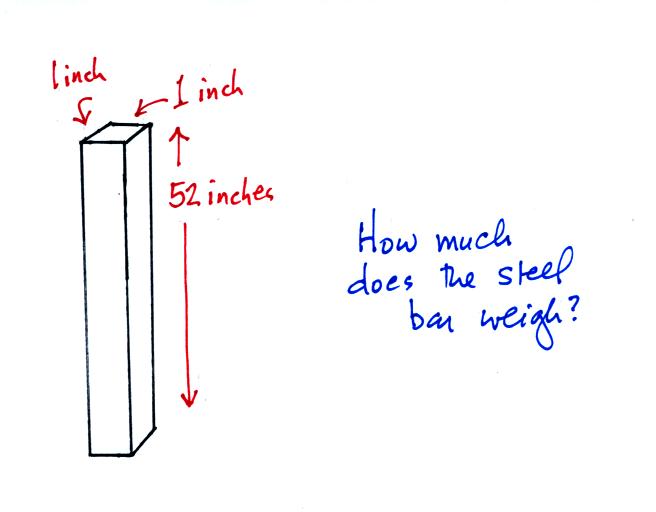
is something you can feel so I passed an iron
bar around in class (it's sketched
below). You were supposed to estimate
it's weight. The fact that it was 1" by
1" is significant. More about the bar
later in today's notes.
A couple of small
plastic bottles were passed during class. One
contained some water the other an equal volume
of mercury (here's the source
of the nice photo of liquid mercury below at
right). I wanted you to appreciate how much
heavier and denser mercury is than water.
Thanks
for being careful with the mercury. A spill would
have shut down the classroom and perhaps more of the
building until the hazardous materials people could come
in and clean it up. It isn't so much the liquid
mercury that is a hazard, but rather the mercury
vapor. Mercury vapor is used in fluorescent bulbs
(including the new energy efficient CFL bulbs) which is
why they need to be disposed of carefully. That is
something we'll mention again later in the class.
I am hoping that you will remember and understand the
following statement
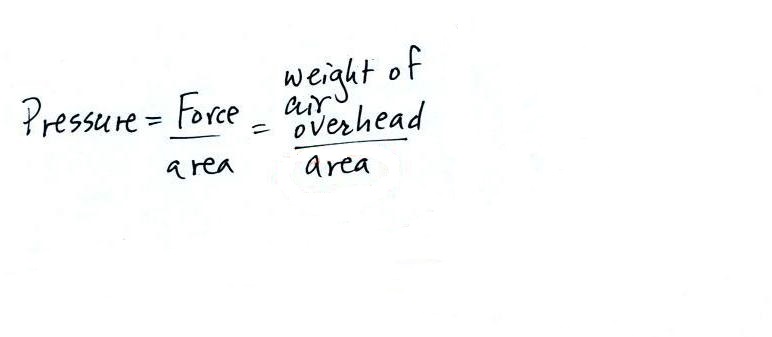
atmospheric
pressure at any level in the atmosphere
depends on (is determined by)
the weight
of the air overhead
We'll
first review the concepts of mass, weight, and
density. I've numbered the various sections
(there are a total of 7) to help with
organization. There's also a summary at the end
of today's notes.
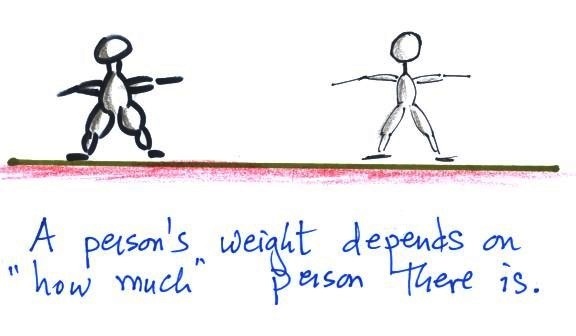
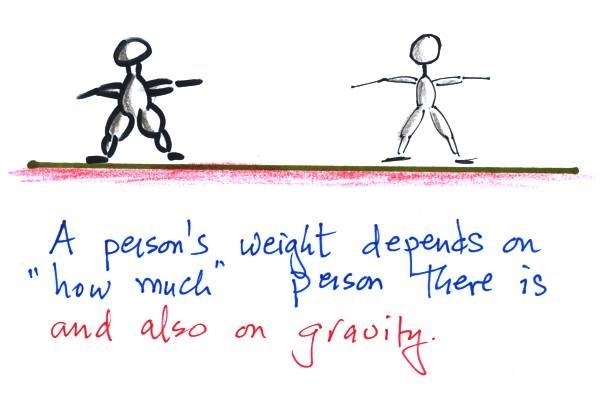
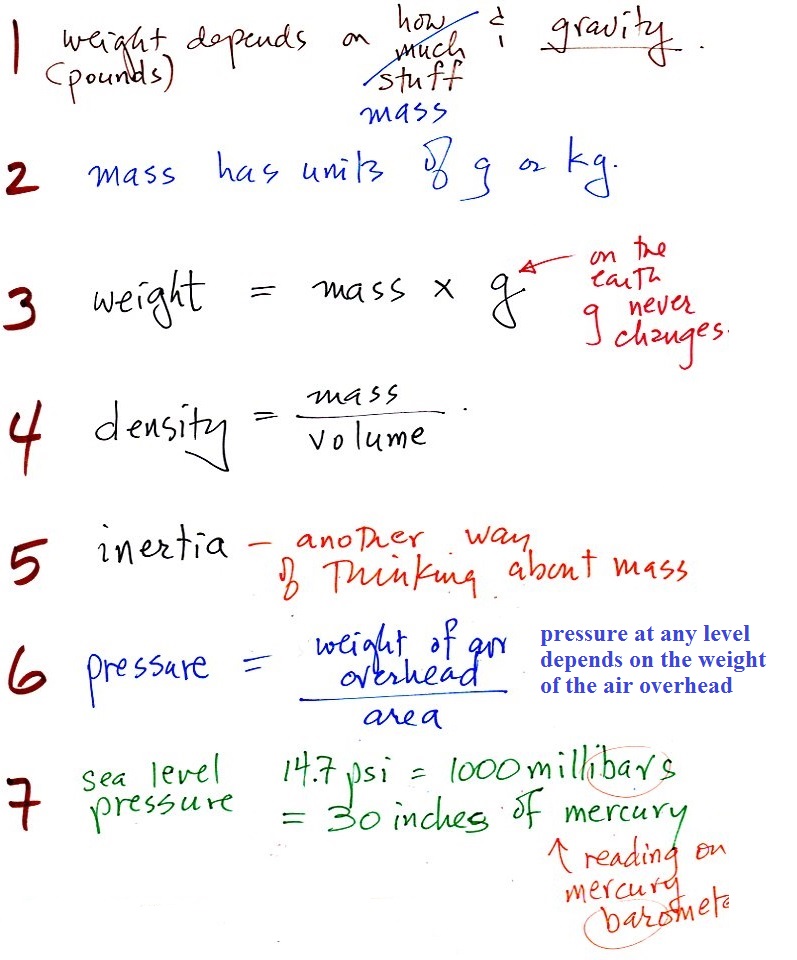
1.
weight
A good place to start because we are most familiar
with this term. We can feel weight and we
routinely measure weight.
A person's weight also depends
on something else.
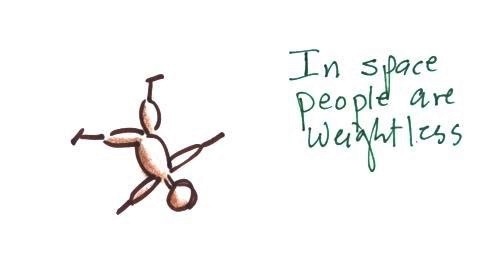
In outer space away from
the pull of the earth's gravity people are weightless.
Weight depends on the person and on the pull of gravity.
We
measure weight all
the time.
What units do we
use? Usually
pounds, but
sometimes ounces
or maybe
tons.
2. mass
Rather than just saying the
amount of something it is probably better to use the
word mass
Grams (g) and kilograms (kg) are commonly used units of mass (1
kg is 1000 g).
3. gravitational
acceleration
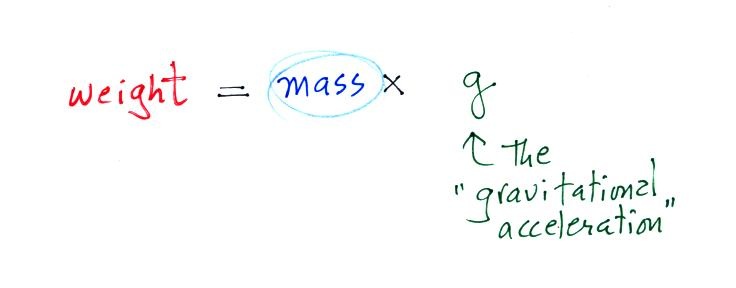
On the surface on the earth, weight is
mass times a constant, g, known as the
gravitational acceleration. The value of g
is what tells us about the strength of gravity on the earth;
it is determined by the size and mass of the earth. On
another planet the value of g would be
different. If you click here
you'll find a little (actually a lot) more information about
Newton's Law of Universal Gravitation. You'll see how
the value of g is determined and why it is called
the gravitational acceleration. These aren't details
you need to worry about but I feel they should be available
in case you're curious.
Here's a question to test your understanding.
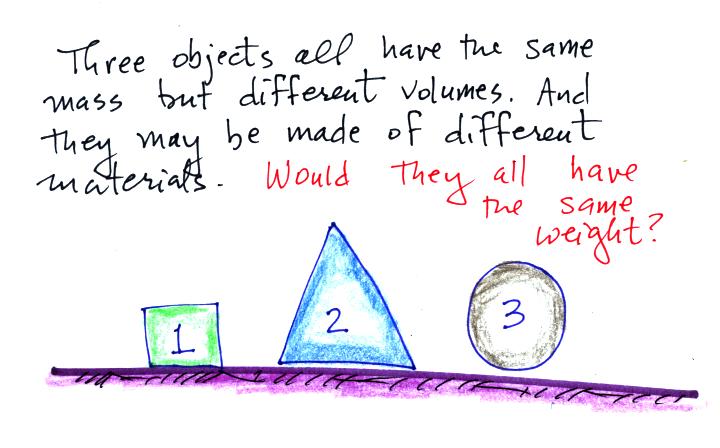
The masses are all the same. On the earth's surface the
masses would all be multiplied by the same value of g.
The weights would all be equal. If all 3 objects had a mass
of 1 kg, they'd all have a weight of 2.2 pounds.
That's why we can use kilograms and pounds interchangeably.
The following figure show a situation where two
objects with the same mass would have different weights.
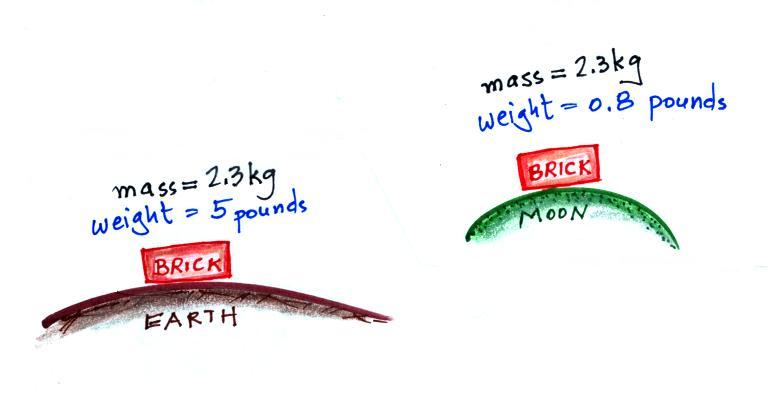
On the earth a brick has a mass of about
2.3 kg and weighs 5 pounds. If you were to travel to the
moon the mass of the brick wouldn't change (it's the same
brick, the same amount of stuff). Gravity on the moon is
weaker (about 6 times weaker) than on the earth because the
moon is smaller, the value of g on the moon is different than
on the earth. The brick would only weigh 0.8 pounds on
the moon. The brick would weigh almost 12
pounds on the surface on Jupiter where gravity is stronger
than on the earth.
The three objects below
were not passed around class (one of them is pretty
heavy). The three objects all had about
the same volumes. One is a piece of wood,
another a brick, and the third something else.
The
easiest way to determine which is which is to lift each
one. One of them weighed about 1 pound (wood), the 2nd
about 5 pounds (a brick) and the last one was 15 pounds (a
block of lead).
The point of all this was to get you thinking about
density. Here we had three objects of about
the same size with very different weights. That means
the objects had different masses (since weight depends on
mass). The three different masses, were squeezed
into roughly the same volume producing objects of very
different densities.
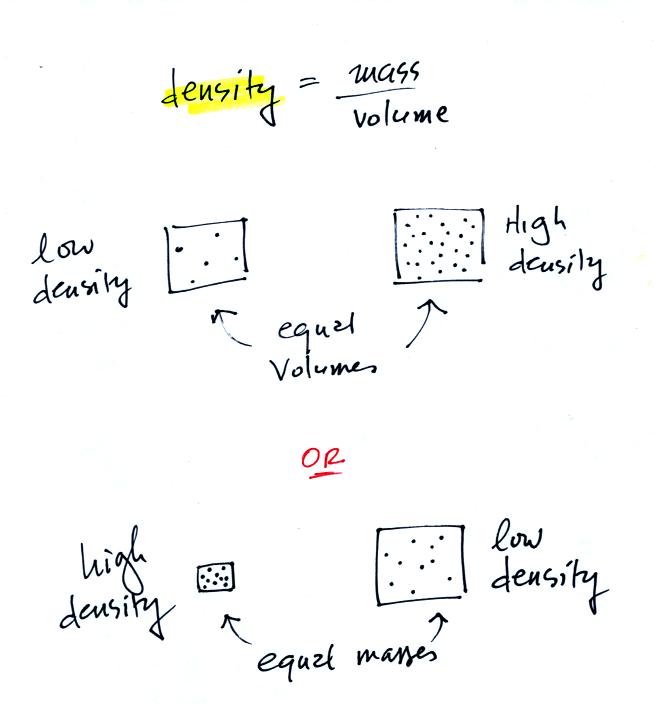
4. density
The brick is in the back, the lead
on the left, and the piece of wood on the right.
The wood is less dense than water (see the table below) and
will float when thrown in water. The brick and the lead
are denser than water and would sink in water.
We'll be more concerned with air in this
class than wood, brick, or lead.
In the first example
below we have two equal volumes of air but the amount in
each is different (the dots represent air
molecules).
The amounts of air (the masses) in the second example are the
same but the volumes are different. The left example
with air squeezed into a smaller volume has the higher
density.
material
|
density g/cc
|
air
|
0.001
|
redwood
|
0.45
|
water
|
1.0
|
iron
|
7.9
|
lead
|
11.3
|
mercury
|
13.6
|
gold
|
19.3
|
platinum
|
21.4
|
iridium
|
22.4
|
osmium
|
22.6
|
g/cc = grams per cubic centimeter
cubic centimeters are units of volume - one cubic
centimeter is about the size of a sugar cube
I wish I could get my hands on a block of iridium or
osmium just to be able to feel how heavy it would be -
it's about 2 times denser than lead.
Here's a more subtle concept. What if we were in outer
space with the three wrapped blocks of lead, wood, and
brick. They'd be weightless.
Could we tell them apart then? They would still have very
different densities and masses but we wouldn't be able to feel how
heavy they were.
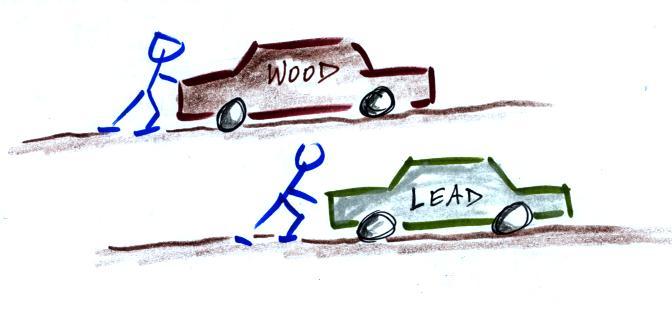
5.
inertia
I think the following illustration will
help you to understand inertia.
Two
stopped cars. They are the same size except one is
made of wood and the other of lead. Which would be
hardest to get moving (a stopped car resists being put
into motion). It would take considerable force to
get the lead car going. Once the cars are moving
they resist a change in that motion. The lead car
would be much harder to slow down and stop.
This is the way you could try to distinguish
between blocks of lead, wood, and brick in outer space.
Give them each a push. The wood would begin moving more
rapidly than the block of lead even if both are given
the same strength push.
I didn't
mention it in class, but this concept of inertia
comes from Newton's 2nd law of motion
F = m a
F is force, m is mass, and a is acceleration. We can
rewrite the equation
a = F/m
This shows cause and effect more clearly. If you exert a
force (cause) on an object it will accelerate (effect).
Acceleration can be a change in speed or a change in direction (or
both). Because the mass is in the denominator, the
acceleration will be less when mass (inertia) is large.
We were starting to run short of time at this point. But
here where we're at
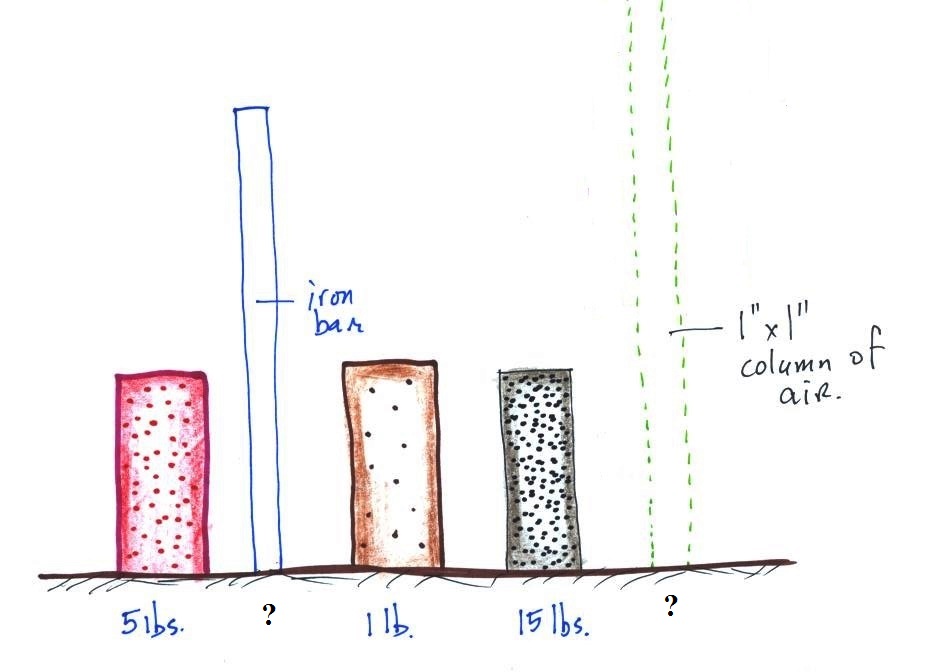
The weight of the iron bar is still unknown.
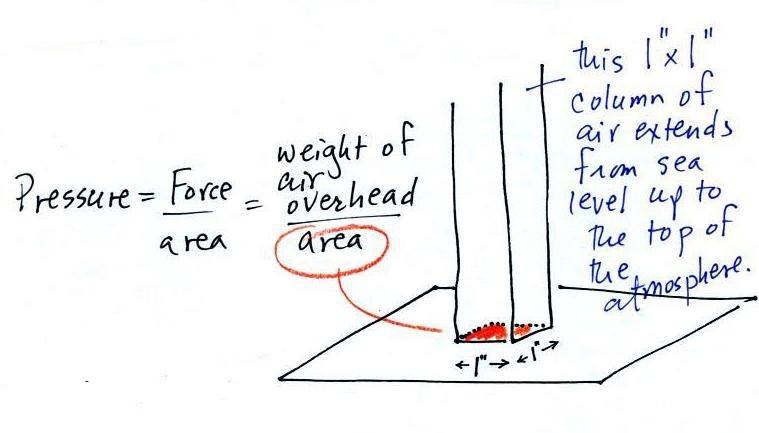
A
very tall 1 inch x
1 inch column of
air has been added
to the
picture.
Other than being a
gas, being
invisible, and
having much lower
density it's
really no
different from the
other objects.
Now
we're ready to
define (and
hopefully
understand)
pressure.
It's a pretty
important
concept.
A lot of what
happens in the
atmosphere is
caused by
pressure
differences.
Pressure
differences
cause
wind.
Large pressure
differences
(such as you
might find in
a tornado or a
hurricane) can
create strong
and
destructive
storms.
The air that
surrounds the earth has mass. Gravity pulls downward on
the atmosphere giving it weight. Galileo conducted a
simple experiment to prove that air has weight (in the
1600s). The experiment wasn't mentioned in
class.
6. pressure
Atmospheric pressure at
any level in the atmosphere
depends on (is determined
by)
the weight of the air
overhead
This
is one way, a sort of large, atmosphere size scale
way, of understanding air pressure.
Pressure depends on, is determined by, the weight of the
air overhead. To determine the pressure you need to
divide the weight by the area it is resting on.
and here we'll apply the
definition to a column of air stretching from sea
level to the top of the atmosphere
Pressure is defined as force divided by area. Atmospheric
pressure is the weight of the air column divided by the area at
the bottom of the column (as illustrated above).
Under normal conditions a 1 inch by 1 inch column of air
stretching from sea level to the top of the atmosphere will weigh
14.7 pounds.
Normal atmospheric pressure at sea level is 14.7 pounds per square
inch (psi, the units you use when you fill up your car
or bike tires with air).
Now back to the iron bar. A lot of people
felt it weighed more than 15 pounds. I don't think
anyone thought is was lighter than 15 pounds. A couple
of people though it might weigh exactly 15 pounds. Turns
out those two were correct. The bar actually weighs
14.7 pounds. When you stand the bar on end, the pressure
at the bottom would be 14.7 psi.
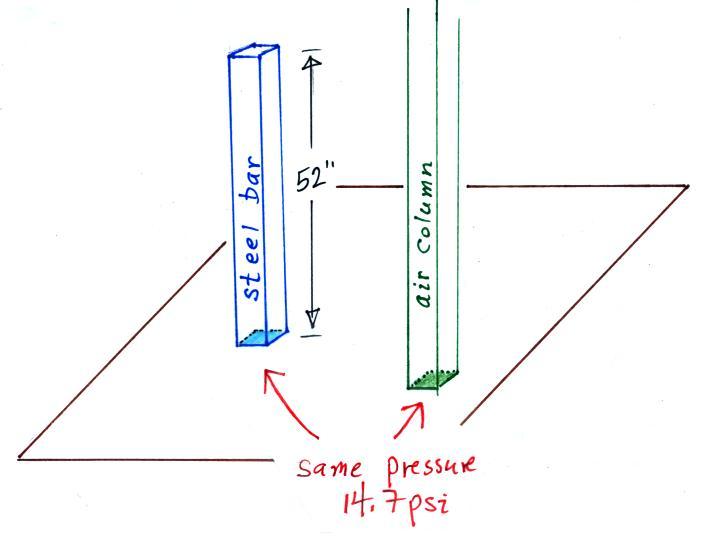
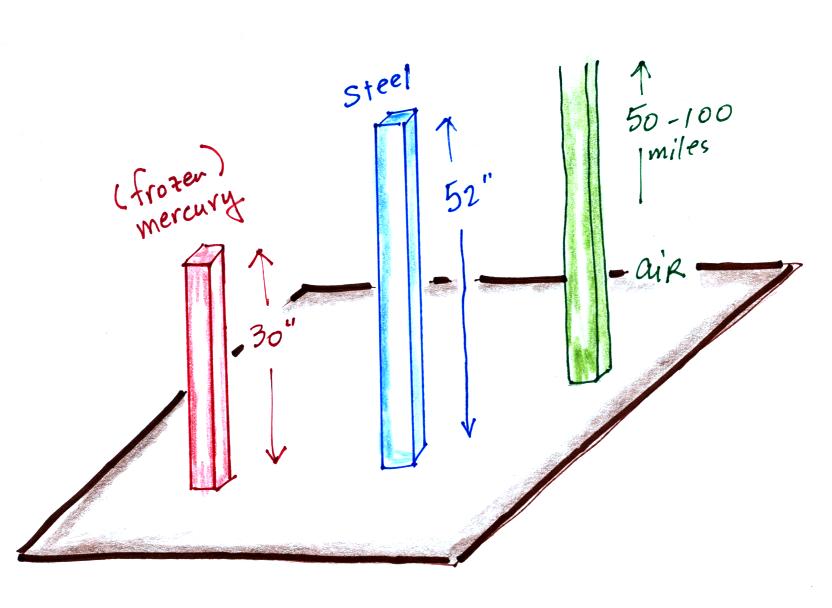
The weight of the 52 inch
long 1" x 1" steel bar is the same as a 1" x 1" column
of air that extends from sea level to the top of the
atmosphere 100 or 200 miles (or more) high. The
pressure at the bottom of both would be 14.7 psi.
7. pressure units
Pounds per square inch, psi, are
perfectly good pressure units, but they aren't the ones
that most meteorologists use.
Typical sea
level pressure is 14.7 psi or about 1000 millibars
(the units used by meteorologists and the units that we will
use in this class most of the time) or about 30 inches of
mercury. Milli means 1/1000 th. So
1000 millibars is the same as 1 bar. You sometimes see
typical sea level pressure written as 1 atmosphere.
Inches
of mercury refers to the reading on a mercury
barometer. This seems like unusual
units for pressure. But if you remember the chart
earlier, Mercury (13.6 grams/cm3)
is denser than steel ( about 7.9 grams/cm3 ). If we could some
how construct a 1" x 1" bar of mercury it would only need to
be 30 inches long to equal the weight or the iron bar or the
weight of a tall column of air.
Each of these columns would weigh 14.7 pounds. The
pressure at the base of each would be the same.
A mercury barometer is, we'll find, just a balance.
You balance the weight of a very tall column of air with the
weight of a much shorter column of (liquid) mercury.
This is as far as we got in class
today (actually a figure or two beyond were we got). We'll
come back to it again next Tuesday.
As promised, here's a short summary of the main points from the
mass, weight, density, and pressure section. This wasn't
shown or mentioned in class.