Tuesday Oct. 6, 2015
Music from Dessa: "Skeleton
Key", "Mineshaft
II", "Dixon's
Girl", and "Sadie
Hawkins".
Listen
to Dessa discuss what it takes to write and sing songs
of this style.
The Experiment #2 reports are due one week from today.
You should try to return the materials this week so that you can
pick up the Supplementary Information handout. You can
either bring them to class or come by my office (PAS 588).
The outdoor door will be open most of the day and you can just
drop off the materials and pick up the handout at your convenience
(it is not necessary to come only during official office hours).
The 1S1P Surface Weather Map Analysis was collected today.
The Upper
Level Charts Optional Assignment is due before the start of
class on Thursday.
A preliminary version of the Quiz #2
Study Guide is now available online. Quiz #2 is
Thursday Oct. 15. Reviews will be held next week, see the
Study Guide for times and locations.
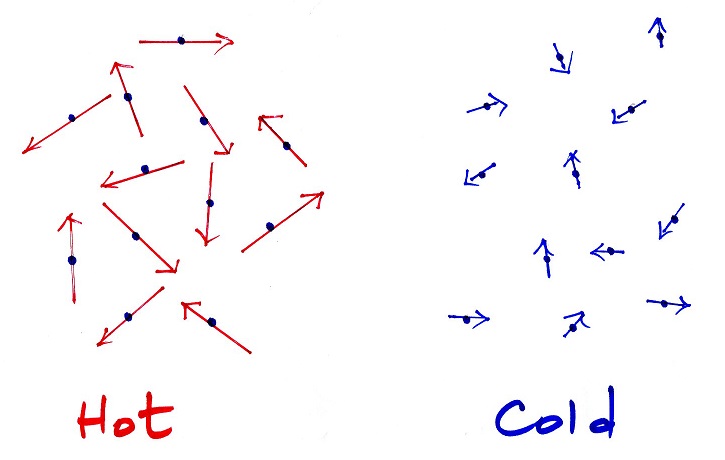
Quick review: temperature & heat, temperature
scales
When you add energy to something its temperature
usually increases. The figure below shows you what
happens inside an object when it's temperature changes.
The atoms or
molecules inside the warmer object will be moving more
rapidly (they'll be moving freely in a gas, just
"jiggling" around while still bonded to each other in a
solid). Temperature provides a measure of
the average
kinetic energy of the atoms or molecules in a
material. Temperature gives you an idea of the
average speed of the moving atoms or molecules in a
material.
You need to be careful what temperature scale you
use when using temperature as a measure of average kinetic
energy. You must use the Kelvin temperature scale
because it does not go below zero (0 K is known as
absolute zero). The smallest kinetic energy you can have
is zero kinetic energy. There is no such thing as
negative kinetic energy.
You can think of heat (heat energy) as
being the total
kinetic energy of all the molecules or atoms in a
material. This
is illustrated below. The figure was drawn so that all of
the atoms or molecules had about the same average kinetic
energy. There are fewer atoms or molecules in the figure at
left. So the total of all the kinetic energies is less than
in the figure at right.
The atoms or
molecules in the examples below have the same
temperatures,
the same average kinetic energies
|
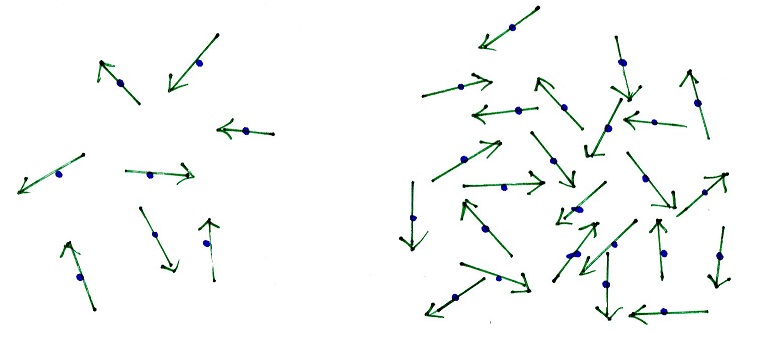 |
The total kinetic energy of
all the atoms or molecules is lower in this example.
|
More atoms or molecules
means the total kinetic energy, the heat energy, is
this example is higher.
|
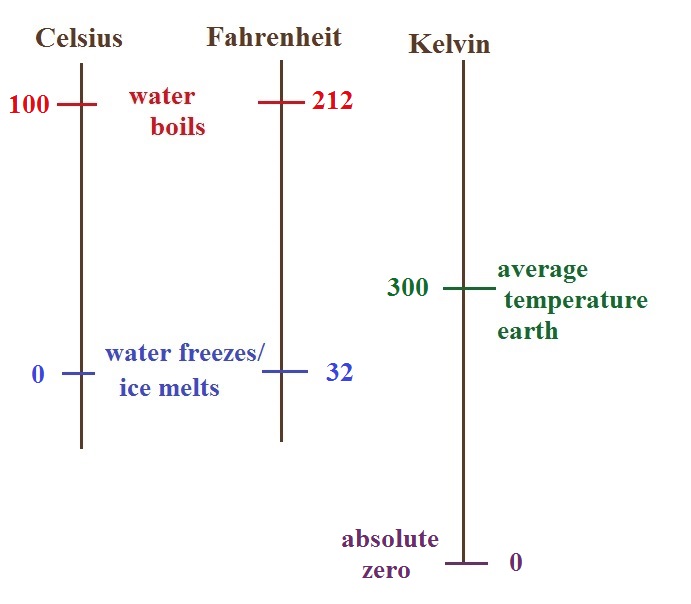
There are three temperature scales that we might have
occasion to use in this class. They're shown below.
There are two temperatures that you should try to remember for
each scale.
The boiling and freezing points of water on
both the Celsius and the Fahrenheit scales (the freezing point of
water and the melting point of ice are the same). Remember
that the Kelvin scale doesn't go below zero. 0 K is referred
to as absolute zero, it's as cold as you can get. A nice
round number of the average temperature of the earth is 300 K,
that's the last temperature value to remember.
Here's some additional temperature data that I didn't show or mention in class.
I'm including it just in case you're interested.
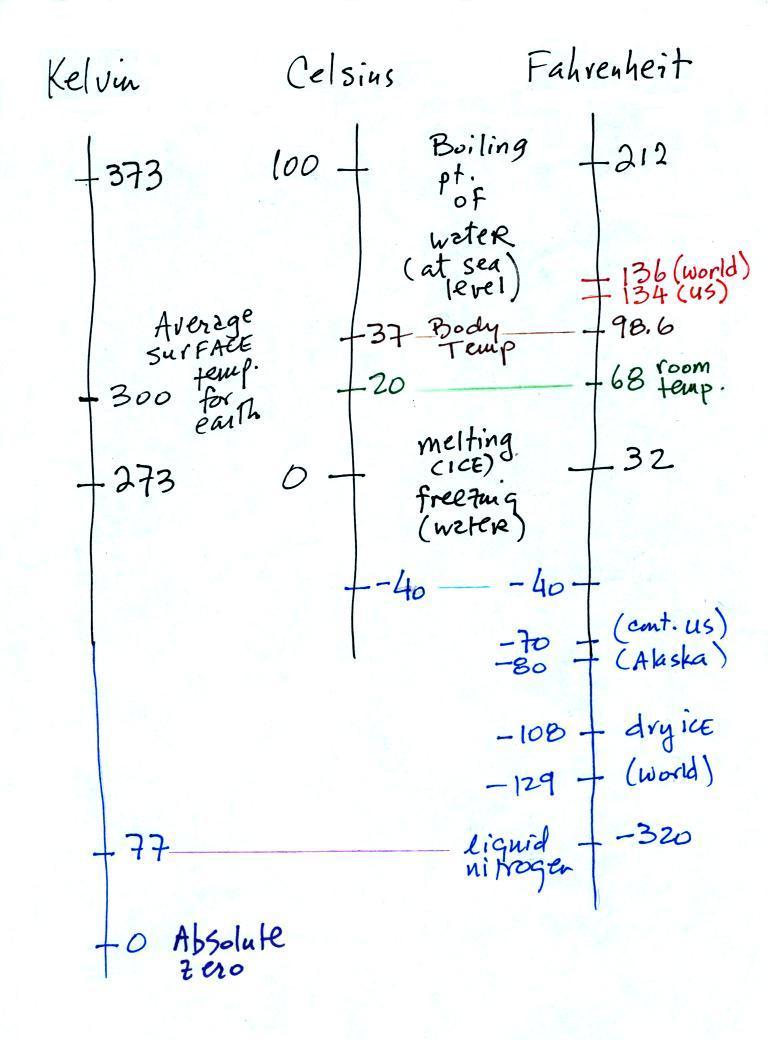
You certainly don't need to try to remember
all these numbers. The world high temperature record value
of 136 F above was measured in Libya at a location that was only
about 35 miles from the Mediterranean coast. Water, as we
have seen, moderates climate so it seemed odd that such a high
temperature would have been recorded there. The World
Meteorological Organization recently decided the 136 F reading
was invalid and the new world record is the 134 F measurement
made in Death Valley.
The continental US cold temperature record of -70 F was set
in Montana and the -80 F value in Alaska. The world record
-129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high
latitude, high altitude, and location in the middle of land
rather than being near or surrounded by ocean (again water
moderates climate, both hot and cold).
Liquid nitrogen is very cold but it is still quite a bit
warmer than absolute zero. Liquid helium gets within a few
degrees of absolute zero, but it's expensive and there's only a
limited amount of helium available. So I would feel guilty
bringing some to class and I don't think it would look any
different than liquid nitrogen.
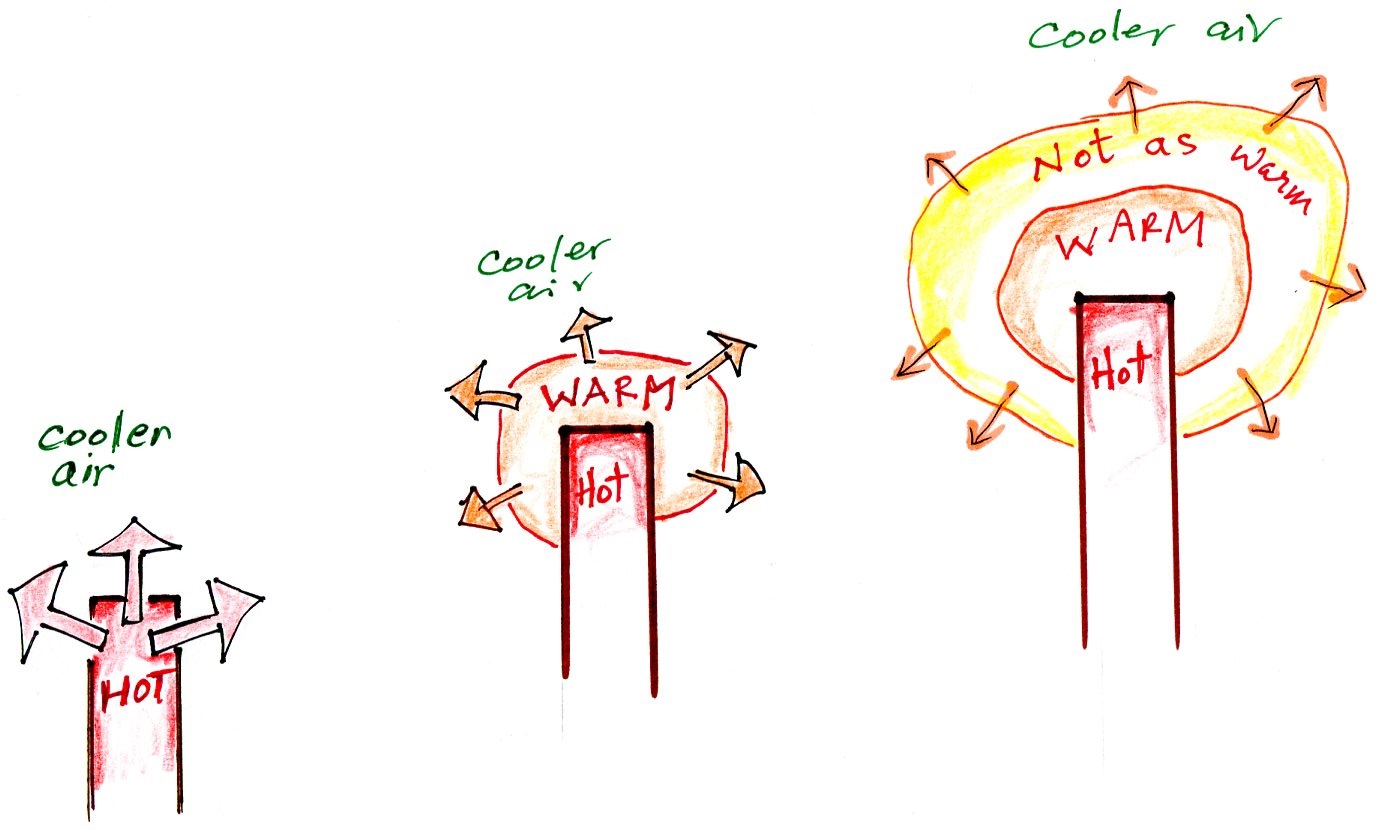
Energy transport by conduction
Conduction is the first of four energy
transport processes that we will cover (and the least
important transport process in the atmosphere). The
figure below illustrates this process. Imagine heating
the end of a piece of copper tubing just so you can visualize
a hot object. If you held the object in air it would
slowly lose energy by conduction and cool off.
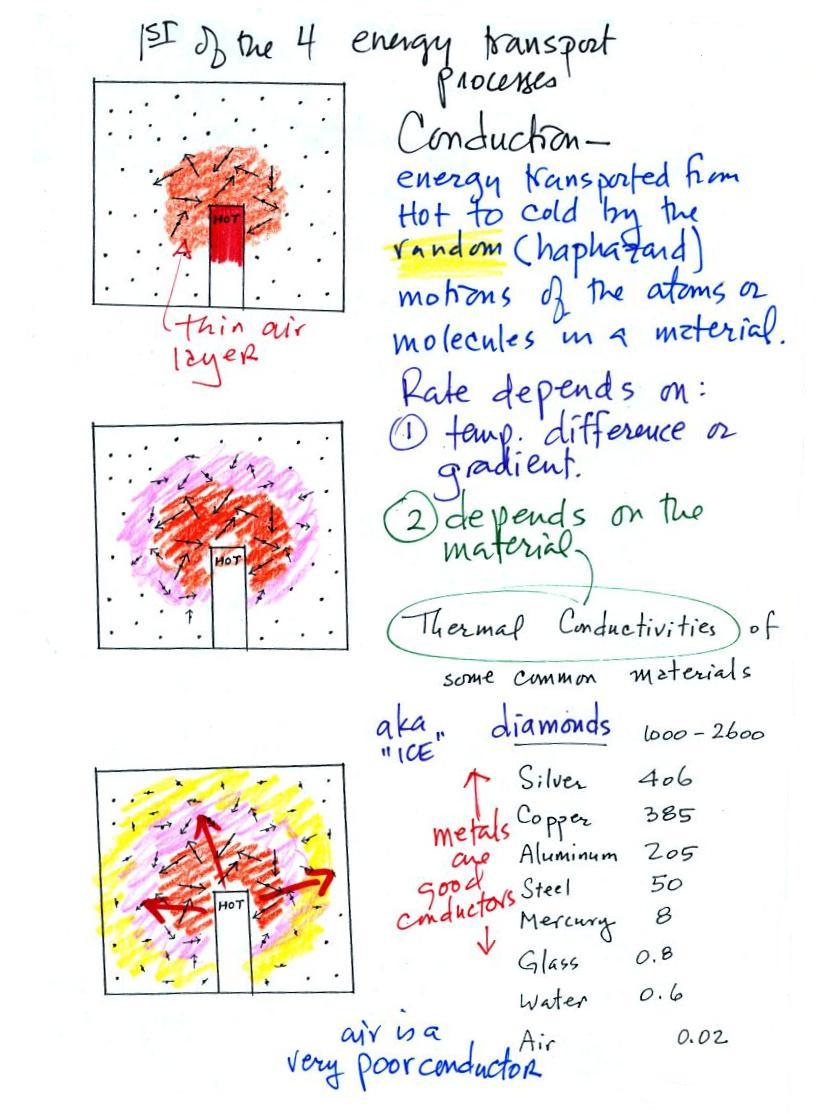
How does that happen? In the top
picture some of the atoms or molecules near the hot object
have collided with the object and picked up energy from the
object. This is reflected by the increased speed of
motion or increased kinetic energy of these molecules or
atoms (these guys are colored orange).
In the middle picture the initial layer of energetic
molecules have collided with some of their neighbors and
shared energy with them (these are pink). The neighbor
molecules have gained energy though they don't have as much
energy as the molecules next to the hot object.
In the third picture molecules further out (yellow) have
now gained some energy. The random motions and
collisions between molecules is carrying energy from the hot
object out into the colder surrounding air.
Conduction transports energy from hot to cold. The
rate of energy transport depends first on the temperature
gradient or temperature difference between the hot object
and the cooler surroundings. If the object in the
picture had been warm rather than hot, less energy would
flow and energy would flow at a slower into the surrounding
air. If there were no temperature difference there
wouldn't be any energy transport at all.
The rate of energy transport also depends on the
material transporting energy (air in the example
above). Thermal conductivities of some common
materials are listed. Air is a very poor conductor of
energy and is generally regarded as an insulator.
Water is a little bit better conductor. Metals are
generally very good conductors (cooking pans are often made
of stainless steel but have aluminum or copper bottoms to
evenly spread out heat when placed on a stove).
Diamond has a very high thermal conductivity (apparently the
highest of all known solids). Diamonds are sometimes
called "ice." They feel cold when you touch
them. The cold feeling is due to the fact that they
conduct energy very quickly away from your warm fingers when
you touch them.
Here's another neater sketch of conduction that was shown
in class.
I brought a propane torch (2 of them actually, one to serve
as a backup) to class to demonstrate the behavior of materials
with different thermal conductivities. Here's
what I wanted to illustrate

|

|

|
Copper is a good
conductor. You must move your fingers several
inches away from the end to keep from getting burned.
|
Glass has much lower thermal
conductivity. You can hold onto the glass just a
couple of inches from the flame and not feel any
heat. Because energy is not being carried away
from the end of the piece of glass, the glass can get
hot enough to begin to glow red.
|
You can put your finger
alongside the flame with just 1/2 inch or so of
separation. Air is a very poor conductor.
Don't put your finger above the flame though.
|
Transport of energy by conduction is similar to the transport
of a strong smell throughout a classroom by diffusion. Small
eddies of wind in the classroom blow in random directions and move
the odor throughout the room. For a demonstration you need
something that has a strong smell but is safe to breathe.
I've tried a variety of things such as curry powder and Vicks
VapoRub in the past. This semester I tried some
garlic. The classroom is too large and the ventilation
system too efficient so the smell doesn't get very far. The
demonstration is still instructive, I think, because you can
visualize what should happen.
Also we can add something
else to the demonstration that might help you to
understand the difference between energy transport by
conduction and by convection.
Because air has such a low thermal conductivity it is often
used as an insulator.
It is important, however, to keep the air trapped in small pockets
or small volumes so that it isn't able to move and transport
energy by convection (we'll look at convection momentarily).
Here are some examples of insulators that use air:
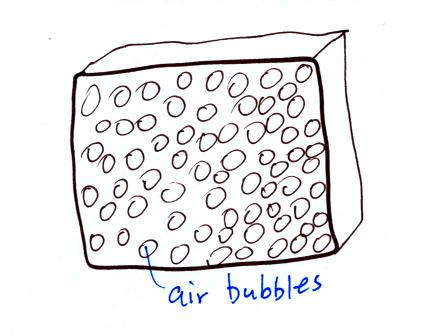
Foam is often used as an
insulator. Foam is filled with lots of small
air bubbles, that's what provides the insulation.
You
can safely hold onto a foam cup filled with liquid nitrogen (-320 F)
because the foam does such a good job insulating
your fingers from the cold liquid inside.
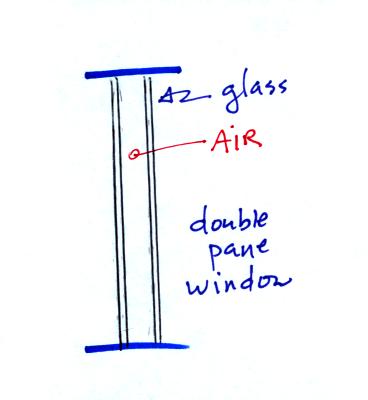
Thin insulating layer of air in a double pane
window. I
don't have double pane windows in my house.
As a matter of fact I leave a window open so my
cats can get in and out of the house (that's not
particularly energy efficient). It also
means there are lots of mosquitoes in the house in
the summer.
We really
haven't needed winter coats yet in Tucson this
semester (rain coats yes but not winter coats).

|

|
| Down feathers are
often used in coats and sleeping bags.
Packing together a bunch of the "clusters"
produces very good insulation provided the
feathers stay "fluffed up" and trap
air. source
of this image |
Synthetic fibers (Primaloft -
Synergy are shown above in a microphotograph) have
some advantages over down. There is still
some insulation when wet and the material is
hypoallergenic. source
of this image |
 |

|
A
photograph of aerogel (image source),
sometimes known as solid air. It's an
excellent insulator because it is mostly
air. The small particles in the
aerogel are scattering light in the same way air
molecules do. That's why it has this sky blue
color.
|
A scanning
electron microscope photograph of asbestos
which was once widely used as insulation.
Asbestos fibers can cause lung cancer and
other damage to your lungs when inhaled.
The white bar at the top left edge of the
image is 50 um across.
You can find this image and read
more about asbestos here.
|
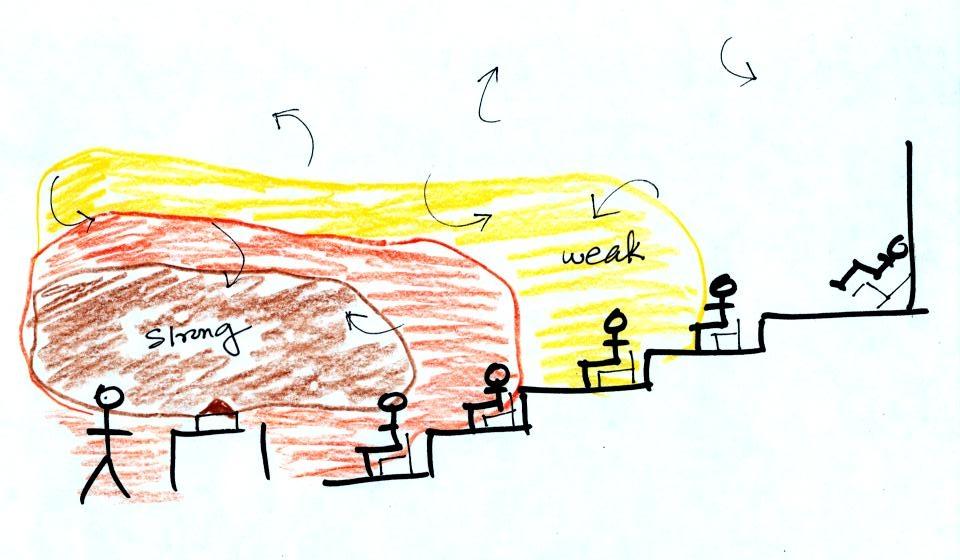
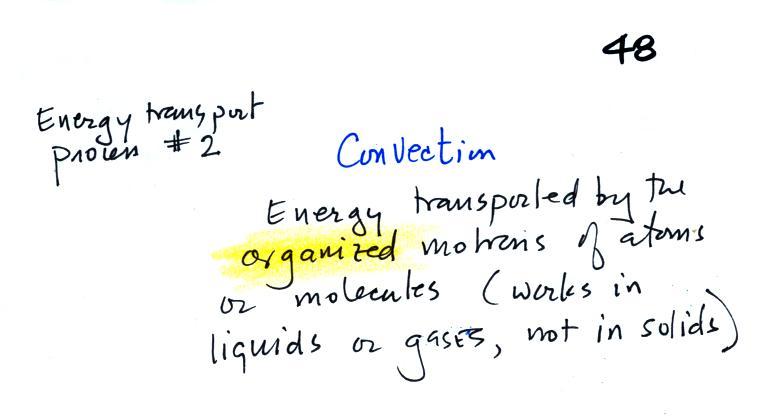
Energy transport by convection
I used the torch to heat up the broken glass graduated cylinder
again. The glass gets so hot that you can see it starting to
glow red.
How would you cool off a hot object like this? You could
just hold onto it and it would eventually cool by
conduction. If you were in a little bit more of a
hurry you could blow on it. That's forced convection,
the energy transport process we will be covering next. Or
you could stick the hot end of the cylinder into some water (you'd
hear a short hissing sound and the glass would probably
shatter). The hissing would mean the hot piece of glass had
evaporated some water. That would be an example of latent
heat energy transport which we'll be discussing later in the
period.
Rather than moving about randomly, the atoms or molecules move
together as a group (organized motion). Convection works in
liquids and gases but not solids (the atoms or molecules in a
solid can't move freely).
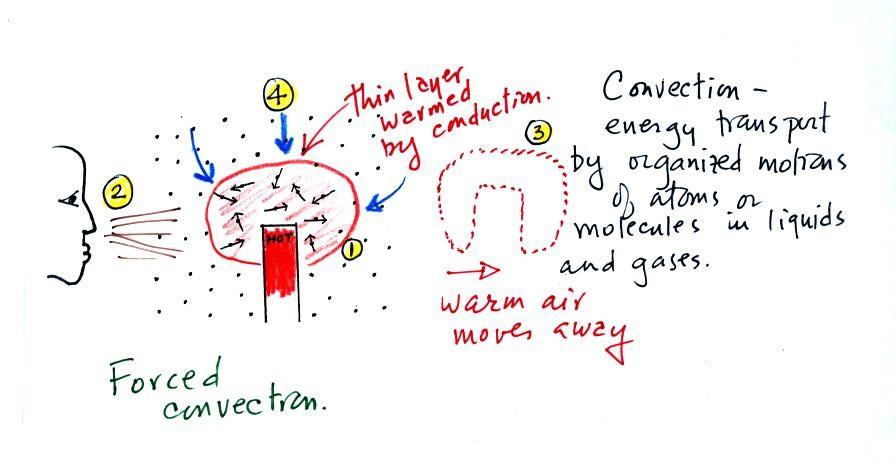
At Point 1 in the picture above
a thin layer of air surrounding a hot object has been heated by
conduction. Then at Point 2 a person is blowing the blob of warm
air off to the right. The warm air molecules are moving
away at Point 3 from the hot object together as a group (that's
the organized part of the motion). At Point 4 cooler air
moves in and surrounds the hot object and the whole process
repeats itself.
Think back to garlic demonstration
earlier in class. Diffusion alone wasn't able to spread
the smell very far into the classroom. To try to spread
the smell somewhat further, we could put a small fan behind the
ground up garlic powder and try to blow the smell further into
the classroom. That would be more like forced convection
and would be more effective than just diffusion.
And actually you don't need to force convection, it will often
happen on its own.
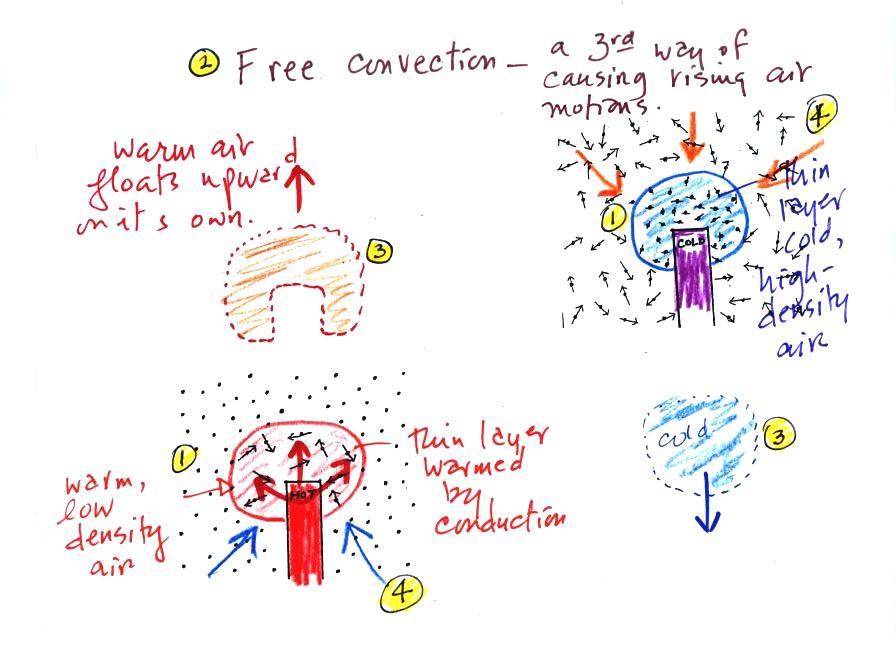
A thin layer of air at Point 1
in the figure above (lower left) is heated by conduction.
Then because hot air is also low density air, it actually isn't
necessary to blow on the hot object, the warm air will rise by
itself (Point 3). Energy is being transported away from
the hot object into the cooler surrounding air. This is
called free convection. Cooler air moves in to take the
place of the rising air at Point 4 and the cycle repeats itself.
The example at upper right is also free convection. Room
temperature air in contact with a cold object loses energy and
becomes cold high density air. The sinking air motions that
would be found around a cold object have the effect of
transporting energy from the room temperature surroundings to the
colder object.
In both examples of free convection, energy is being
transported from hot toward cold.
I could put my finger alongside the flame from the propane
torch without any problem. There's very little energy
transported sideways through air by conduction.

Be careful if you put your
finger or hand above the torch. That's
because there's a lot of very hot air rising from the
torch. This is energy transport by free convection
and its something you can sometimes see.
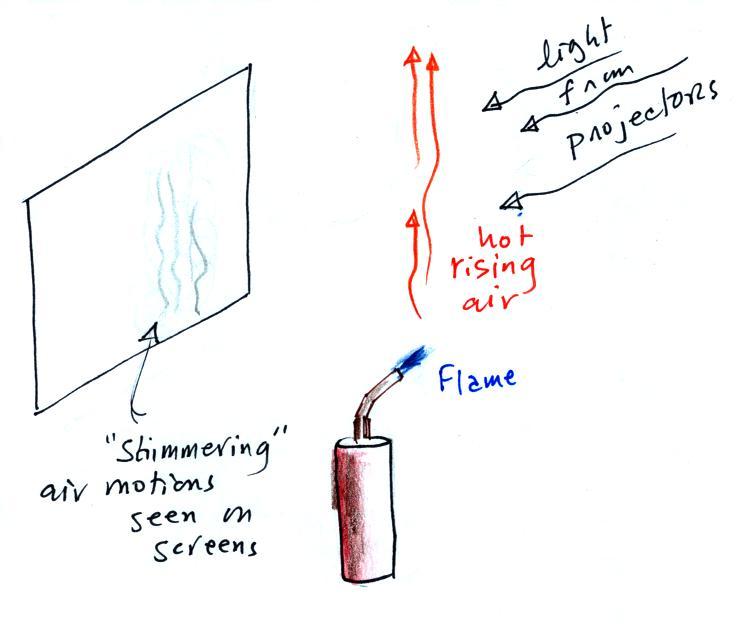
Up at the front of the classroom you might have
been able to see (barely) the
shimmering of hot rising air when I held the torch in
front of the projector screen. There is a
technique, called Schlieren photography, that can
better catch these barely visible air motions (it is
able to see and photograph the differences in air
density). The photo at right is an example and
shows the hot rising air above a candle.
The photo was taken by Gary Settles from Penn
State University and can be found at this
site.
Now some
surprisingly practical applications, I think, of what we have
learned about conductive and convective energy
transport. Energy transport really does show up in a lot
more everyday real life situations than you might expect.
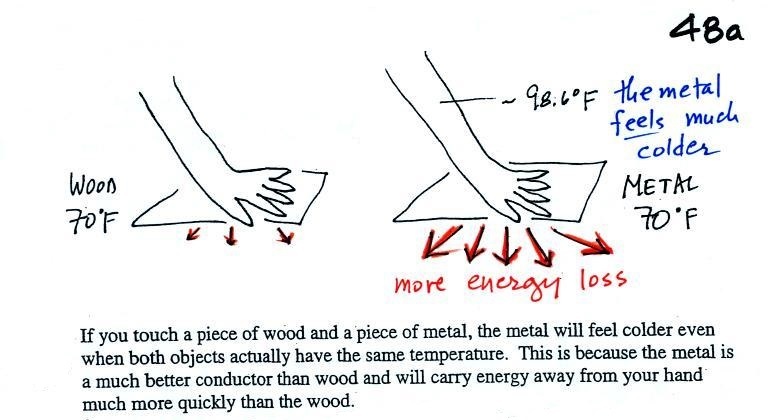
Note first of all there is a temperature difference between
your hand and a room temperature (70 F) object. Energy
will flow from your warm hand to the colder object.
Metals are better conductors than wood. If you touch a
piece of 70 F metal it will feel much colder than a piece of
70 F wood, even though they both have the same
temperature. A piece of 70 F diamond would feel even
colder because it is an even better conductor than
metal. A piece of aluminum and a piece of wood (oak)
were passed around class so that you could check this out for
yourself.
Something that feels cold may not be as cold as it seems.
Our
perception of cold
is more an indication of how quickly our
body or hand is losing energy
than a reliable measurement of
temperature.
Here's another
example
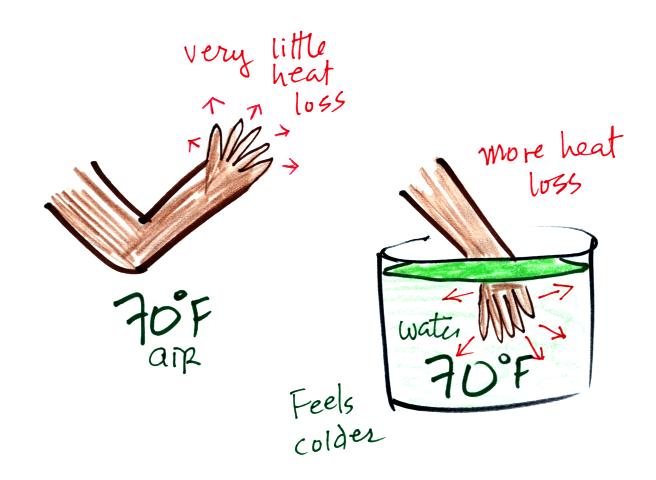
It's pleasant standing outside on a
nice day in 70 F air, it doesn't feel warm or cold.
But if you jump into 70 F pool water you will feel cold,
at least until you "get used to" the water temperature
(your body might reduce blood flow to your extremities and
skin to try to reduce energy loss).
Air is a poor conductor. If you go out in 40 F
weather you will feel cold largely because there is a
larger temperature difference between you and your
surroundings (and temperature difference is one of the
factors that affect rate of energy transport by
conduction).
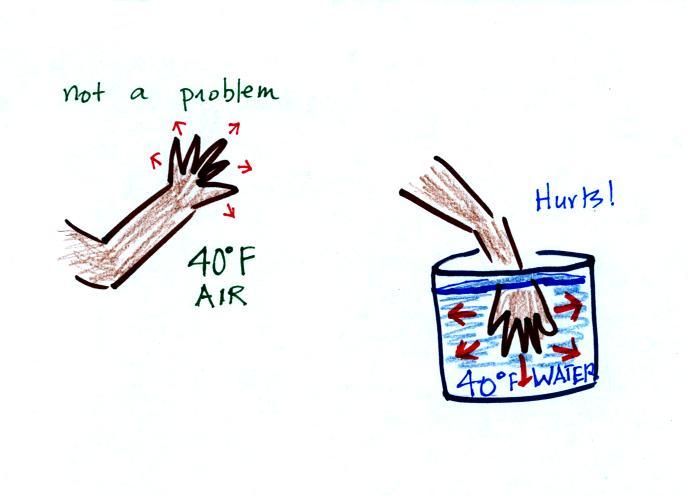
If you stick
your hand into a bucket of 40 F water, it will feel very
cold (your hand will actually soon begin to hurt).
Keep some warm water nearby to warm up your hand.
Water is a much better conductor than air.
Energy flows much more rapidly from your hand into the
cold water. I mentioned in class that I thought this
might be good for you. The reason is that successive
application of hot and then cold is sometimes used to
treat arthritis
joint pain (it used to work wonders for my Dad's
knee).
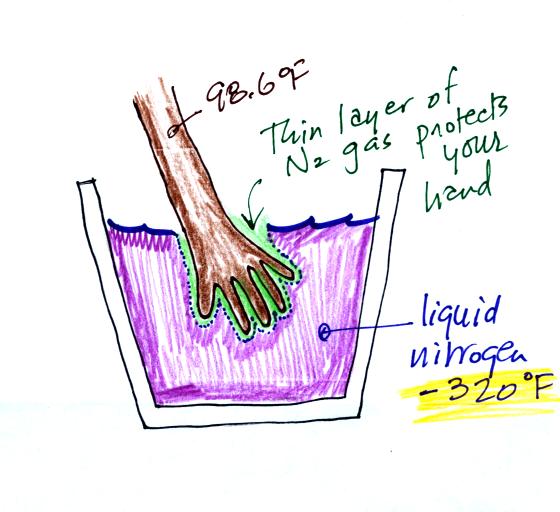
You can safely stick your hand
into liquid nitrogen for a fraction of a
second. There is an enormous temperature
difference between your hand and the liquid nitrogen
which would ordinarily cause energy to leave your
hand at a dangerously high rate (which could cause
your hand to freeze solid). It doesn't feel
particularly cold though and doesn't feel wet.
The reason is that some of the liquid nitrogen
evaporates and quickly surrounds your hand with a
layer of nitrogen gas. Just like air, nitrogen
is a poor conductor (air is mostly nitrogen).
The nitrogen gas insulates your hand from the cold
for a very short time (the gas is a poor conductor
but a conductor nonetheless) If you leave your
hand in the liquid nitrogen for even a few seconds
it would freeze. That would cause irreparable
damage.
A question came up in class a few semesters ago
about sticking you hand (or maybe just the tip of
one finger) into molten lead. I've never seen
it done and certainly haven't tried it myself.
But I suspected that you would first need to wet
your hand. Then once you stick it into the
lead the water would vaporize and surround your hand
with a thin layer of gas, water vapor. The
water vapor is a poor conductor just like the
nitrogen and oxygen in air, and that protects your
hand, for a short time, from the intense heat.
Here's a
video (and water does play a critical role)
Wind chill
Wind chill is a really good example of energy transport by
convection. As a matter of fact I'm hoping that if I
mention energy transport by convection that you'll first
think of wind chill. Wind chill is also a reminder
that our perception of cold is an indication of how
quickly our body is losing energy rather than an accurate
measurement of temperature.
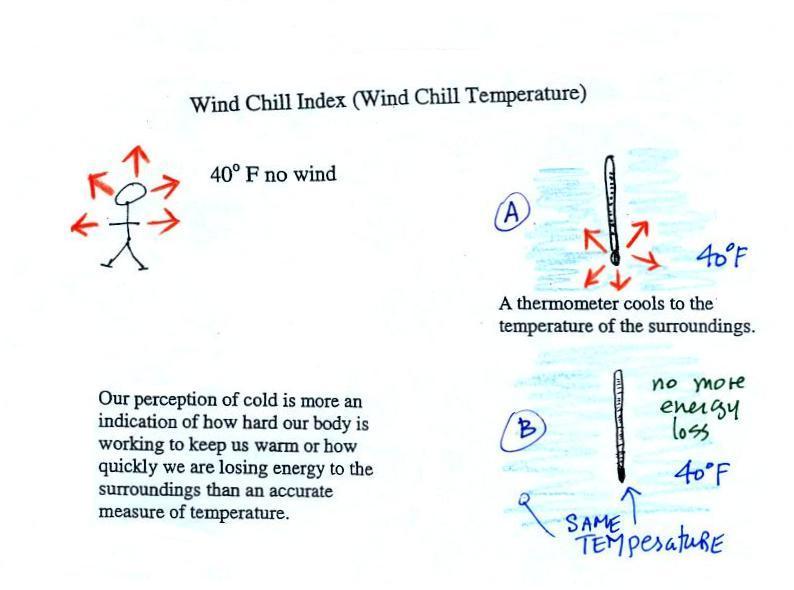
Your body works hard to keep its
core temperature around 98.6 F. If
you go outside on a 40 F day (calm winds) you will
feel cool; your body is losing energy to the colder
surroundings (by conduction mainly). Your body
will be able to keep you warm for a little while
(perhaps indefinitely, I don't know). The 5
arrows represent the rate at which your body is
losing energy.
A thermometer behaves
differently, it is supposed to cool to the
temperature of the surroundings. Once it
reaches 40 F and has the same temperature as the air
around it the energy loss will stop. If your
body cools to 40 F you will die.
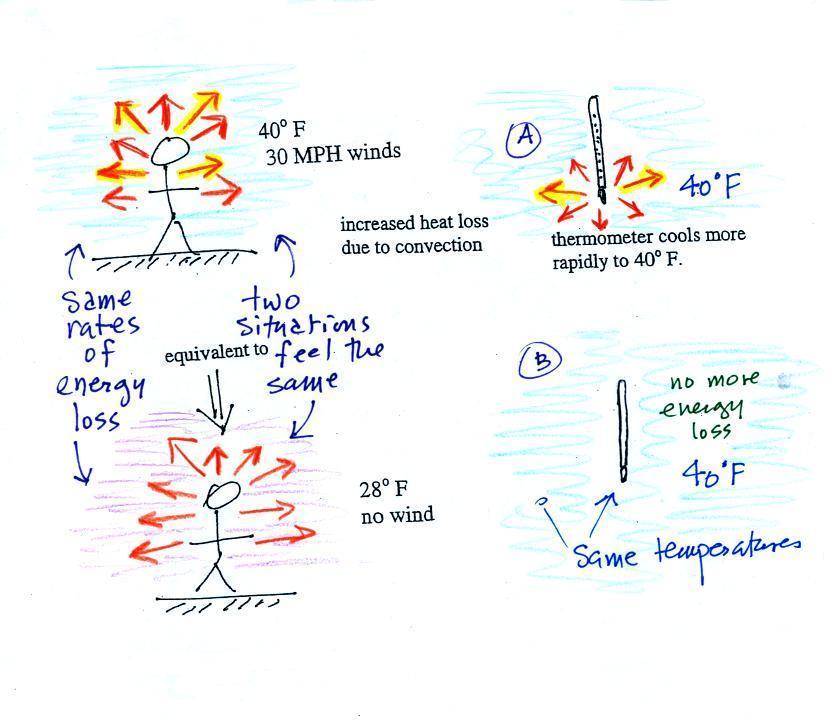
If you go outside on a 40 F day with 30 MPH winds your
body will lose energy at a more rapid rate (because
convection together with conduction are transporting
energy away from your body). Note the additional
arrows drawn on the figures above indicating the greater
heat loss. This higher rate of energy loss will make
it feel
colder than a 40 F day with calm
winds.
Actually, in terms of the rate at which
your body loses energy, the windy 40 F day would feel the
same as a 28 F day without any wind. Your body
is losing energy at the same rate in both cases (9
arrows in both cases). The combination 40 F
and 30 MPH winds results in a wind chill temperature
of 28 F.
You would feel colder on a 40 F day with 30 MPH winds
but the actual temperature is still 40 F. The
thermometer will again cool to the temperature of its
surroundings, it will just cool more quickly on a windy
day. Once the thermometer reaches 40 F there won't
be any additional energy flow or further cooling. The thermometer
would measure 40 F on both the calm and the windy day.
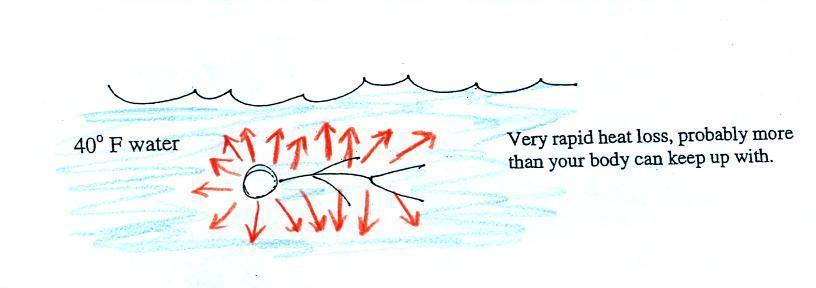
Standing outside on a 40 F day is not an
immediate life threatening situation. Falling
into 40 F water is, you'd last about 30 minutes (you'd
probably go unconscious before that and die by
drowning).
Energy will be conducted away from your
body more quickly than your body can replace it.
Your core body temperature will drop and bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia
which can bring on heatstroke and is a serious
outdoors risk in S. Arizona in the summer.
Talk of how long you would last in 40 F water
reminds me of a page from National Geographic Magazine
that lists some of the limits
of human survival. I can't just scan the
original and add it to the notes without violating
copyright laws. But if you click on the link
above you'll find all of the same information online
in the form of a quiz.
Latent heat energy transport
We spent what little time was left in class rushing
through latent heat energy transport. This
is the 3rd and the next to most important energy transport
process that we will cover. And because we
probably hurried through it more quickly than we should
have you should definitely carefully read through the
following section on your own.
If you had an object that you wanted to cool off
quickly you could blow on it. That might take a
minute or two (maybe more). Or you could stick it
into some water, that would cool it off pretty quickly
because water will conduct energy more rapidly than
air. With a really hot object immersed in water,
you'd probably hear a brief sizzling sound, the sound of
boiling water. A lot of energy would be taken
quickly from the hot object and used to boil (evaporate)
the water. The cooling in this case takes only a few
seconds. It's a very potent energy transport
process.
Latent heat energy transport is sometimes a
little hard to visualize or understand because the energy
is "hidden" in water vapor or water.

Latent heat energy transport involves
changes in phase or state. You should be able to
name each of these phase changes sketched above (this is
p. 55 in the ClassNotes). You should also be able
to indicate whether energy must be added to or removed
from the material in order for each phase change to take
place. For example, do you need to add energy to
ice or take energy from a piece of ice to cause it to
melt.
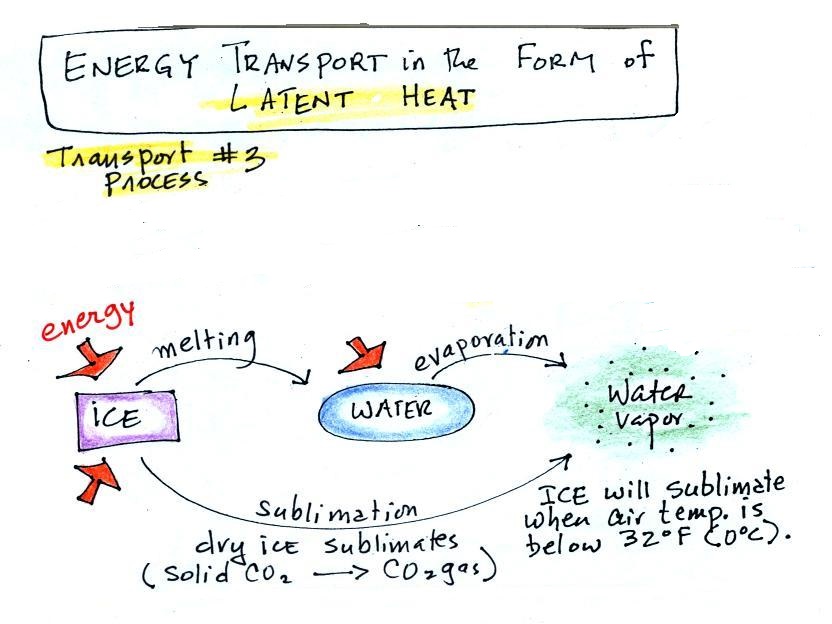
A solid to liquid phase change is
melting, liquid to gas is evaporation, and sublimation
is a solid to gas phase change.
Dry ice is the best example of sublimation that I can
think of. When placed in a warm room, dry ice
turns directly from solid carbon dioxide to gaseous
carbon dioxide without melting first. If you wash
clothes and stick them outside on a cold (below
freezing) day they will eventually dry. The
clothes would first freeze but then the ice would slowly
sublime away.
In each case above energy must be added to the
material changing phase. You can consciously add
or supply the energy (such as when you put water in a
pan and put the pan on a hot stove and cause it to
boil).
That much is pretty clear. The confusing part of
this topic is when phase changes occur without you
playing any role. Energy is still required to melt
ice; in this case the needed energy will be taken
from the surroundings. It is not always obvious
what the "surroundings" are.
Here are a couple of examples
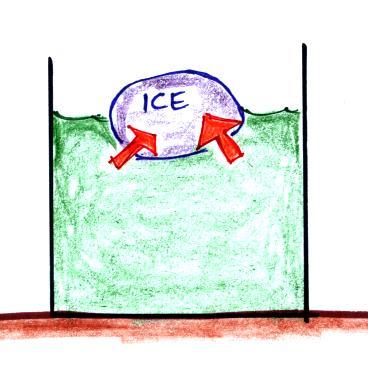
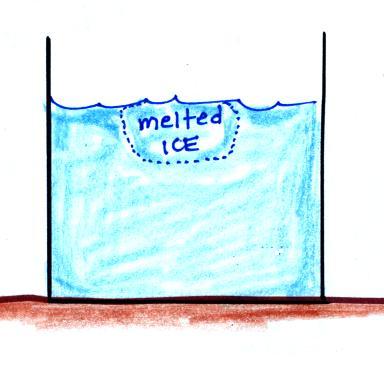
You put an ice cube in a glass of room temperature water.
Energy will naturally flow
from hot to cold; in this case from the water (about 70
F) to the ice (32 F). This transport of energy
would occur via conduction.
Energy is taken from the
water and used to melt the ice. Because energy
is taken from the water, the water cools.
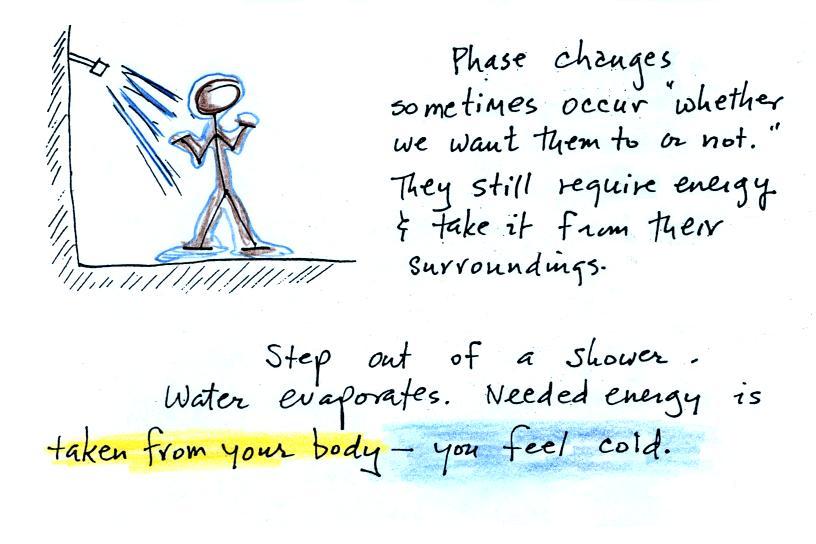
Here's another, maybe even better, example
because it's something you can experience and feel.
When you step out of the shower in the morning you're
covered with water. Some of the water
evaporates. It doesn't ask permission, it just does
evaporates whether you like it or not. The energy
needed for evaporation is taken from the surroundings, the
surroundings in this case are your body. Because your
body is losing energy your body feels cold.
The object of this figure is to give you some
appreciation for the amount of energy involved in phase
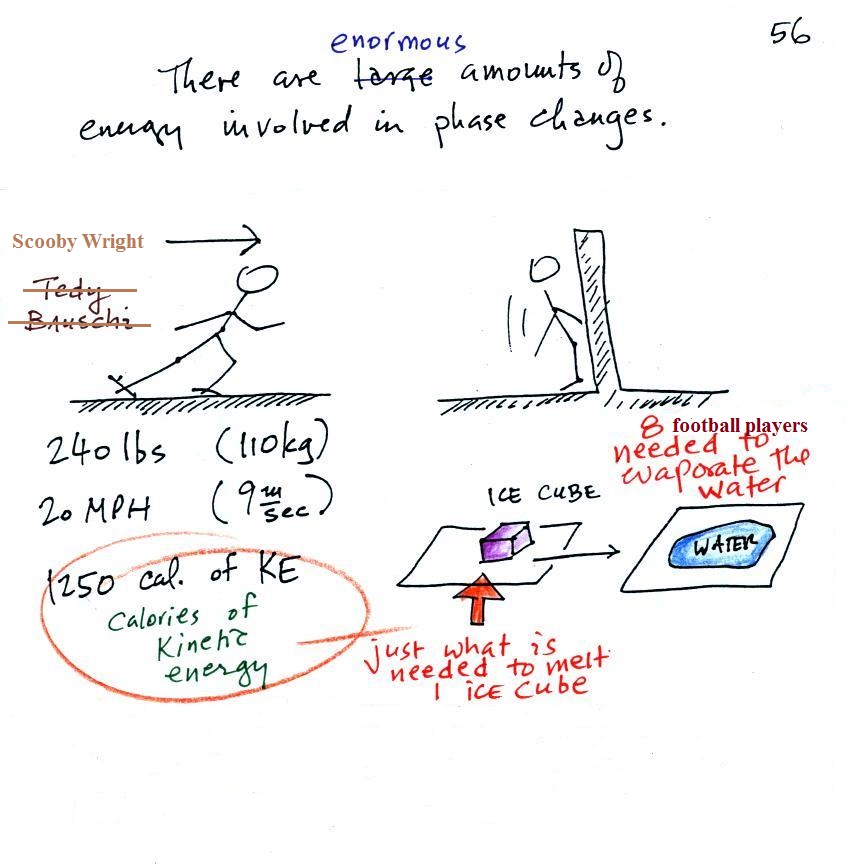
changes. A 240 pound man or woman running at 20 MPH
has just enough kinetic energy (if you could capture it) to
be able to melt an ordinary ice cube (I have been
using Tedy
Bruschi as an example for several years but he's now
retired so I have switched to Scooby Wright). It would
take 8 people running at 20 MPH to evaporate the resulting
ice water.
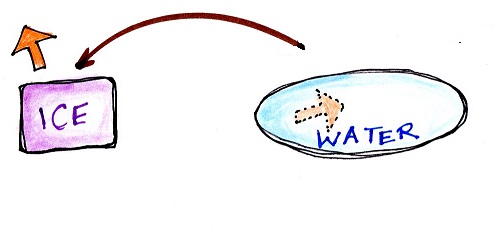
Latent heat energy is energy that is hidden in water
or water vapor. Here's a new
figure appearing for the first time this semester.
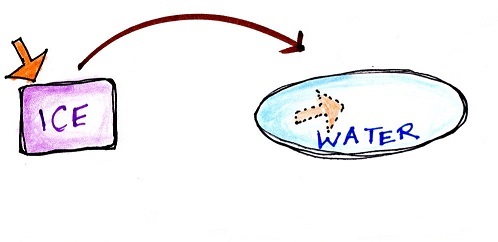
|
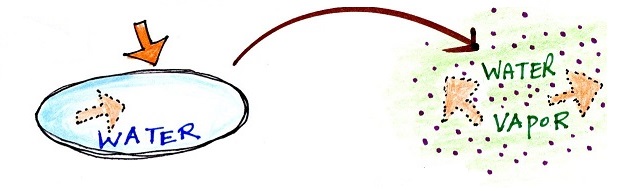
|
Energy added to melt the ice is
hidden in the water that results
|
Energy added to
evaporate the water is added to the energy
already in the water and is hidden in the water
vapor
|
 |
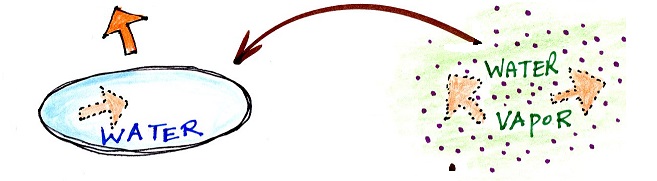
Phase changes can go in the other direction
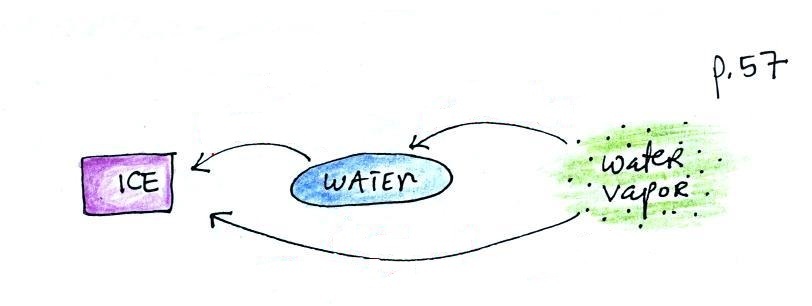
Again, try to name each phase
change and show the direction of energy flow (into or
out of the material) when the phase change occurs
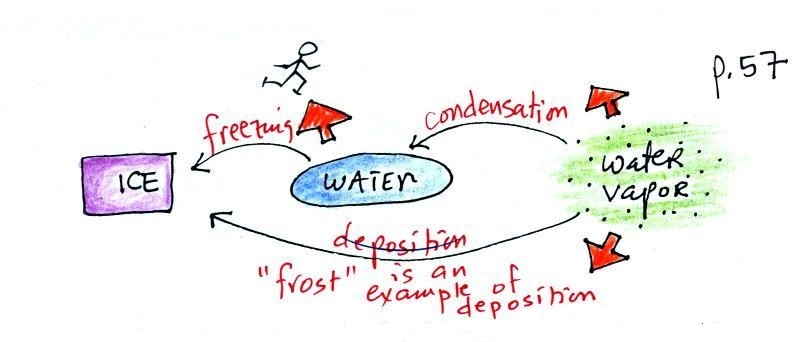
You might not have heard of
deposition before when a gas changes directly to a
solid. The formation of frost is an example of
deposition.
You can consciously remove energy from water vapor to
make it condense. You take energy out of water
to cause it to freeze (you could put water in a
freezer; energy would flow from the relatively
warm water to the colder surroundings). If one
of these phase changes occurs, without you playing a
role, energy will be released into the surroundings
(causing the surroundings to warm).
Note the direction of the energy arrows - energy is
being released into the surroundings (warming the
surroundings). It's kind of like a genie coming
out of a magic lamp. One Scooby Wright's worth
of kinetic energy is released when enough water
freezes to make an ice cube. Many Scooby Wrights
are released when water vapor condenses.
This release of energy into the
surroundings and the warming of the surroundings is a
little harder for us to appreciate because it never
really happens to us in a way that we can feel.
Have you ever stepped out of an air conditioned
building into warm moist air outdoors and had your
glasses or sunglasses "steam up"? Water vapor
never condenses onto your body (your body is too
warm). However if it did you would feel
warm. It would be just the opposite of the cold
feeling when you step out of the shower or a pool and
the water on your body evaporates. You know how
cold the evaporation can make you feel, the same
amount of condensation would produce a lot of warming.
I suspect we'd be surprised at how much warming it
produces.
Alternate view
showing the latent heat energy in water vapor
and water coming out of hiding during a phase
change and being released into the
surroundings.
Here's a practical application of what we have
been learning.
This example wasn't shown or
mentioned in class.
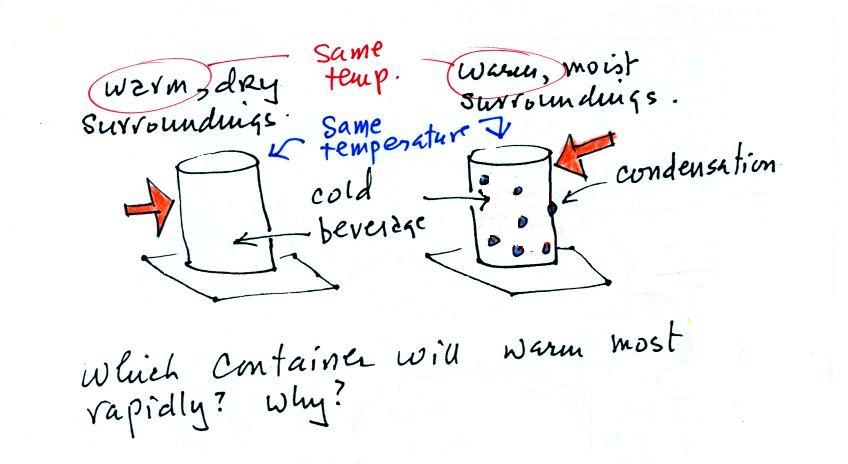
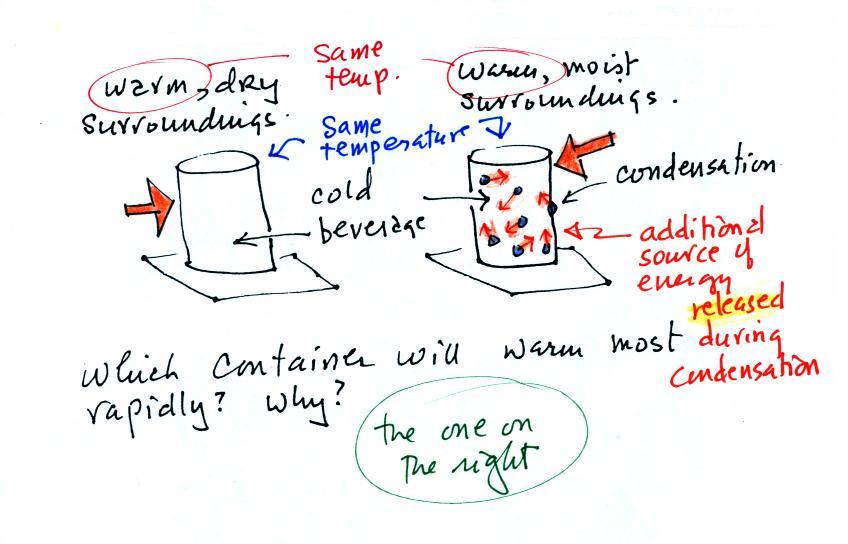
Cans of a cold drink are taken out of the
refrigerator and placed on the kitchen table on a
warm dry day and a warm humid day. Except
for the differences in the amount of moisture in
the air everything else is the same.
Moisture has condensed onto the can above at
right. Do the two cans warm up at the same
rate or does one warm up more quickly than the
other. In the latter case which cans warms
up most rapidly.
The can on the right will warm more quickly.
Equal amounts of heat will flow from the warm air
into the cold cans in both cases.
Condensation of water vapor is an additional
source of energy and will warm that can more
rapidly. I suspect that the condensation may
actually be the dominant process.
The foam "cozy", "koozie",
or whatever you want to call it, that you can put
around a can of soda or beer is designed to
insulate the can from the warmer surroundings but
also to keep water vapor in the air from
condensing onto the can.
It's been a long day
but we're almost done. Two more
figures to illustrate how latent heat energy
transport can carry energy from location to
another.
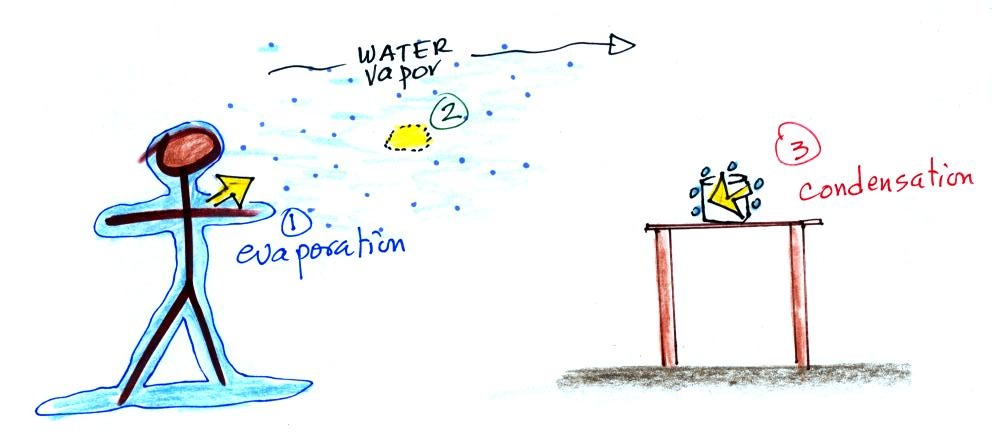
1. You've just stepped out of
the shower and are covered with water. The water
is evaporating and energy is being taken from your
body.
2. The water vapor (containing latent heat
energy, the energy taken from your body), drifts into
the kitchen where it finds a cold can sitting on a
table.
3. Water vapor comes into contact with the cold
can and condenses. The hidden latent heat energy
in the water vapor is released into the can and warms
the drink inside.
Without you even leaving
the bathroom, energy has effectively been
transported from your warm body to a cold can in
the kitchen.
The next figure was not
shown or discussed in class.
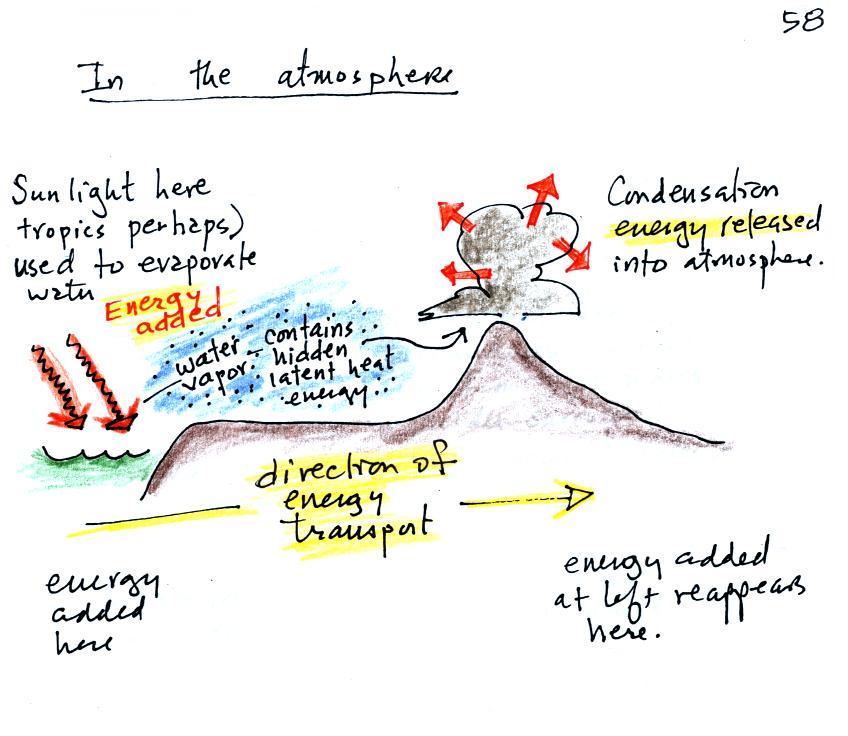
We start in this picture in the
tropics where there is often a surplus of sunlight
energy. Some of the incoming sunlight evaporates
ocean water. The resulting water vapor moves
somewhere else and carries hidden latent heat energy
with it. This hidden energy reappears when something
(air running into a mountain and rising, expanding, and
cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding
atmosphere. This would warm the air.
Energy arriving in sunlight in the tropics has
effectively been transported to the atmosphere in a
place like Tucson.