In order to describe the physics of these processes, we need some way to specify the water vapor content of the atmosphere. There are many ways to do this and we will use a few of them in this class. The first is something called vapor pressure.
Vapor pressure (e) is the pressure (force/area) exerted by water vapor molecules alone. The higher the concentration of water vapor molecules (number density), the higher the vapor pressure. The average air pressure at sea level is about 1013 millibars (mb). If the total air pressure is 1013 mb and water vapor makes up 1% of the air molecules, then the vapor pressure is 1% of 1013 mb or 10.13 mb. Water vapor is a trace gas in the atmosphere of Earth -- the maximum vapor pressure is never more than about 40 mb. For now the important concept is that vapor pressure is one way to keep track of the amount of the gas water vapor. The higher the vapor pressure, the greater the amount of water vapor in the air.
A Physical Explanation of the Processes of Evaporation and Condensation and the concept of Saturation, are provided in this figure, which is also provided as an in-class handout. Evaporation and condensation are competing processes that occur simultaneously. The rate of evaporation, which is the number of water molecules that change phase from liquid to gas each second, depends mainly on the temperature of the liquid water. The higher the temperature of the liquid water, the faster the rate of evaporation. Conversely, the rate of condensation, which is the number of water molecules that change phase from gas to liquid per second, depends mainly on the vapor pressure. The higher the vapor pressure, the faster the rate of condensation. Condensation occurs when a water vapor molecule collides with a liquid water surface, and chemically binds to liquid water molecules. Evaporation occurs when a liquid water molecule attains sufficient energy to escape its chemical bonds with neighboring water molecules.
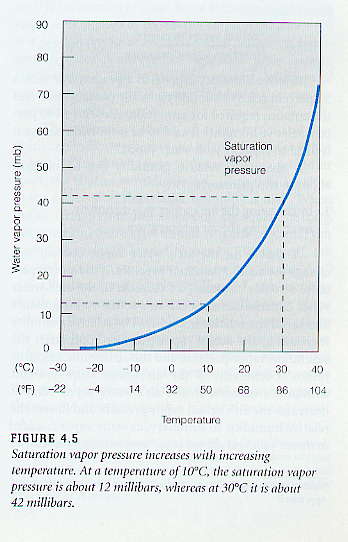
In a closed system, a dynamic equilibrium will be reached, where the rate of evaporation equals the rate of condensation. When this occurs, the the saturation vapor pressure (es ) can be measured, and the air inside the closed system is said to be saturated with water vapor. You may take saturation to mean the capacity or maximum amount of water vapor that will can exist in the air at at given temperature. It is very important to understand that the higher the air temperature, the higher the saturation vapor pressure. Saturation vapor pressure has a strong temperature dependence. As the figure below shows, a small increase in temperature, results in a large increase in the saturation vapor pressure or capacity for water vapor in the air. In other words, warm air can hold more water vapor than cold air. See figure below.
|
Near the Earth's surface, the vapor pressure is usually less than the saturation vapor pressure. (NOTE: the actual vapor pressure of the air can vary from zero up to the saturation vapor pressure). Therefore, the rate of evaporation is greater than the rate of condensation, i.e., liquid water near the Earth's surface is continually evaporating (changing phase from liquid to gas). You can easily convince yourself that this is true by leaving out a glass of water. All of the liquid will eventually evaporate. The reason the air near the ground does not reach saturation is because after the water evaporates, the water vapor is able to move away from the surface. It is not trapped as in the closed experiment described above.
Once water has evaporated it becomes part of the gases that make up the atmosphere. When air rises upward, it cools (the reason rising air cools will be explained later). As air cools, its saturation vapor pressure decreases, in other words, the maximum amount of water vapor that the air can hold decreases. If the air rises high enough and cools sufficiently, it will not be able to hold all the water vapor it contains. When this happens, water vapor must condense back to liquid water. This is how clouds form. Clouds are composed of tiny droplets of liquid water (and possibly ice). Water vapor is an invisible gas and cannot ever be seen. If you see it, e.g., clouds, steam, your breath on a cold day, it must be liquid water droplets. We will talk more about clouds soon.