Tuesday Sept. 18, 2012
click here to
download today's notes in a more printer friendly format
Three songs from The Be Good Tanyas before class this
morning. We started out with "Waiting Around to Die"
which
is something I heard watching an old episode of Breaking
Bad. That was followed by "Rowdy Blues" and
"When
Doves
Cry".
Both Optional
Assignments have been graded and were returned in class
today. If your paper doesn't have a grade it means you earned
full credit. Be sure to check the online answers as not all of
the questions are always graded. Here are answers to
Asst. #1 and here
are answers to the in-class assignment from last Friday.
Quiz #1 is Thursday this week. The quiz will cover material
on both the Quiz #1 Study Guide and the Practice Quiz Study Guide. Reviews are
scheduled for Tuesday and Wednesday afternoon. See either study
guide for times and locations.
The Experiment #1 reports were collected
today. It takes about 1 week to grade the reports. If you
haven't returned your materials please do so as soon as you can.
The graduated cylinders are used in Experiment #2 and need to be
cleaned before they can be checked out. Experiment
#2 materials will be distributed before the quiz on Thursday.
We got a good start on the station model notation last
Thursday. There's one piece of information, pressure, that we
need to learn a little more about.
The problem with the pressure number (highlighted in yellow above)
is that some information is missing. Here's what you need to know
about the pressure data.

Meteorologists hope to map out small horizontal pressure
changes on
surface weather maps (that produce wind and storms). Pressure
changes much more quickly when
moving in a vertical direction. The pressure measurements are all
corrected to sea level altitude to remove the effects of
altitude. If this were not done large differences in pressure at
different cities at different altitudes would completely hide the
smaller horizontal changes.
In the example above, a station
pressure value of 927.3 mb was measured in Tucson. Since Tucson
is about 750 meters above sea level, a 75 mb correction is added to the
station pressure (1 mb for every 10 meters of altitude). The sea
level pressure estimate for Tucson is 927.3 + 75 = 1002.3 mb.
This sea level pressure estimate is the number that gets plotted on the
surface weather map.
Do you need to remember all the
details above and be able to calculate the exact correction
needed? No. You
should
remember that a correction for altitude is needed.
And the correction needs to be added to the station pressure.
I.e. the sea-level pressure is higher than the station pressure.
The calculation above is shown in a picture below

Here are some examples of coding
and decoding the pressure data.
First of all we'll take some sea
level pressure values and show
what needs to be done before the data is plotted on the surface weather
map. These should be the same numbers that we used in
class.
To save room, the leading 9 or 10
on the sea level pressure
value and
the decimal
point are removed before plotting the data on the map. For
example the 10 and the decimal pt in
1002.3 mb would
be removed; 023
would be plotted on the weather map (to the upper right of the center
circle). Some additional examples are shown above.
You'll mostly have to go the other way - read data off a map and
figure out what the sea level pressure is. This is illustrated
below.
When reading pressure values off a
map you must remember to
add a 9 or
10 and a decimal point. For example
118 could be either 911.8 or 1011.8 mb. You
pick the value that
falls closest to 1000 mb average sea level pressure. (so 1011.8
mb would be the correct
value, 911.8 mb would be too low).
Back to the example from the start of class. We can now
decode the pressure information.
Another
important piece of information on a surface map is the time the
observations were collected. We didn't have time
for this in class. Time on a
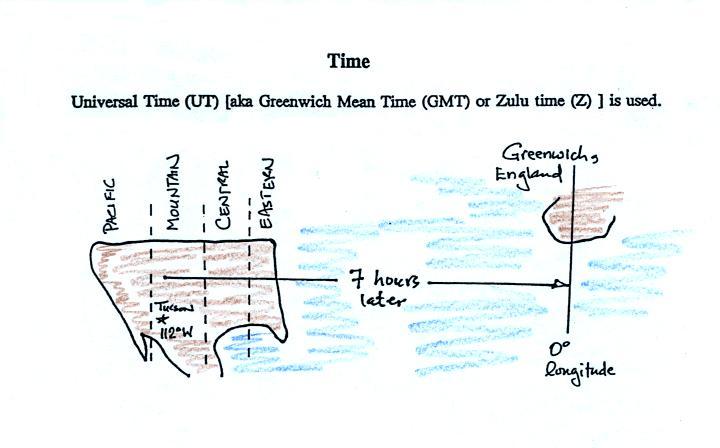
surface map is converted to a universally agreed upon time zone called
Universal Time (or Greenwich Mean Time, or Zulu time).
That is the time at 0 degrees longitude, the Prime Meridian.
There is a 7 hour time
zone difference between Tucson and Universal Time (this
never changes because Tucson stays on Mountain
Standard Time year round). You must add 7
hours to the time in Tucson to obtain Universal Time.
Here are several examples of conversions between MST and UT
to convert from MST (Mountain Standard Time) to UT (Universal Time)
10:20 am MST:
add the 7
hour time zone correction ---> 10:20
+ 7:00 = 17:20 UT (5:20 pm in Greenwich)
2:45 pm MST :
first convert to the 24 hour clock
by adding 12 hours 2:45 pm MST + 12:00 = 14:45 MST
add the 7 hour time zone
correction ---> 14:45 + 7:00 = 21:45 UT (7:45 pm in England)
7:45 pm MST:
convert to the 24 hour clock by
adding 12 hours 7:45 pm MST + 12:00 = 19:45 MST
add the 7 hour time zone correction ---> 19:45 + 7:00 = 26:45 UT
since this is greater than 24:00 (past midnight) we'll subtract 24
hours 26:45 UT - 24:00 = 02:45 am the next day
to convert from UT to MST
18Z:
subtract the 7 hour time
zone
correction ---> 18:00 - 7:00 = 11:00 am MST
02Z:
if we subtract the 7 hour time
zone correction we will get a negative
number.
So we will first add 24:00 to 02:00 UT then subtract 7 hours
02:00
+
24:00
=
26:00
26:00 - 7:00 = 19:00 MST on the previous day
2 hours past midnight in Greenwich is 7 pm the previous day in
Tucson
A bunch of weather data has been
plotted (using the station model notation) on a surface weather map in
the figure
below (p. 38 in the ClassNotes).
Plotting the surface weather
data
on a map is
just the
beginning.
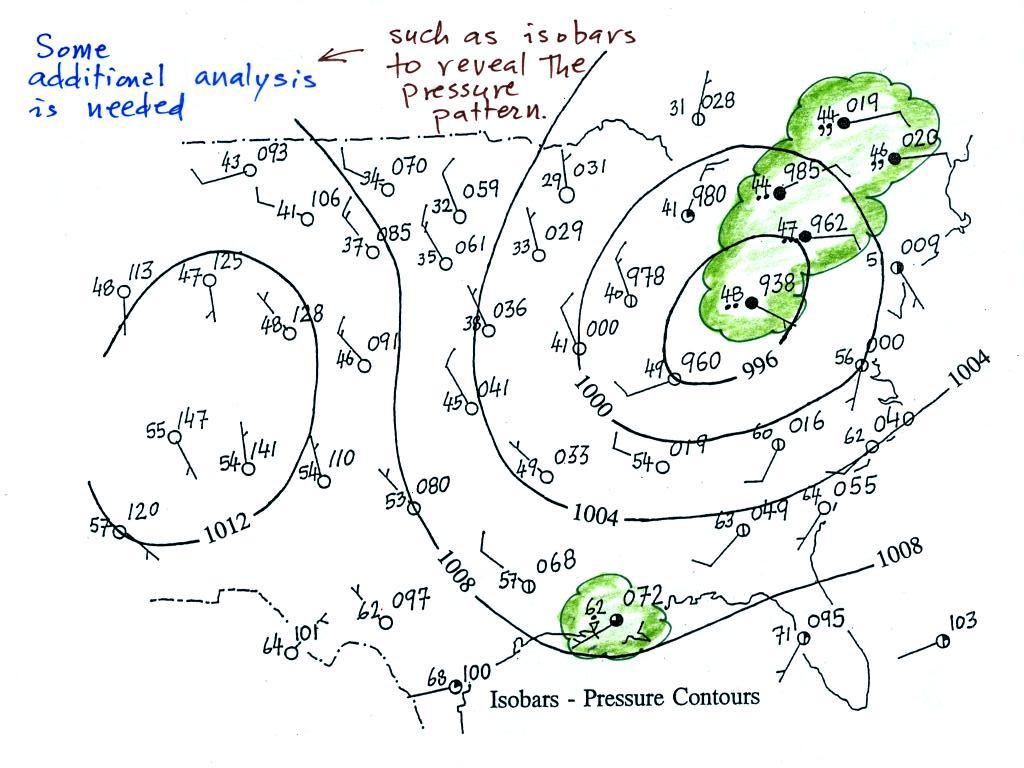
For example you really can't tell what is causing the cloudy weather
with rain (the dot symbols are rain) and drizzle (the comma symbols) in
the NE portion of the map above or the rain
shower along the Gulf Coast. Some additional
analysis is needed. A meteorologist would usually begin by
drawing some contour lines of pressure (isobars) to map out the large
scale
pressure pattern. We will look first at contour lines of
temperature, they are a little easier to understand (the plotted data
is easier to decode and temperature varies across the country in a more
predictable way).
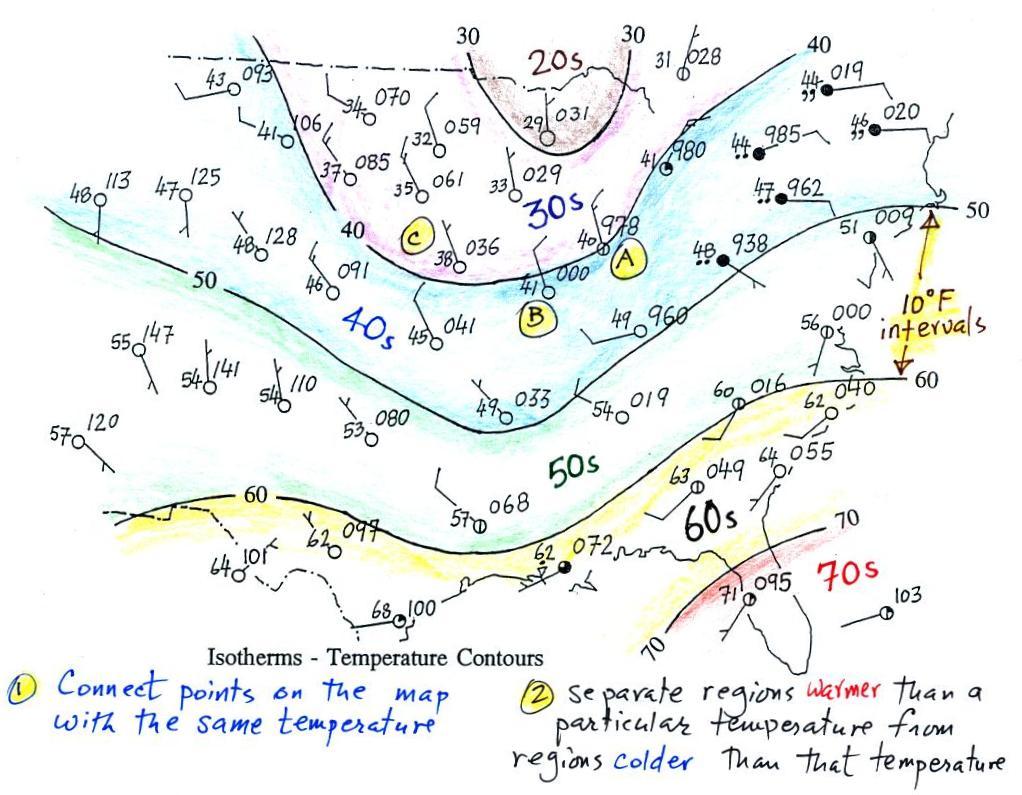
Isotherms, temperature
contour lines, are usually drawn at 10o F
intervals.
They do two things: (1) connect points on the map that all
have the same temperature, and (2) separate regions that are warmer
than a particular temperature from regions that are colder. The
40o F isotherm above passes
through
a city which is reporting a temperature of exactly 40o (Point A).
Mostly
it
goes
between
pairs
of
cities:
one
with
a
temperature
warmer
than
40o (41o at Point B) and
the other
colder
than 40o (38o F at Point C).
Temperatures
generally decrease with
increasing
latitude: warmest temperatures are usually in the south, colder
temperatures in the north.
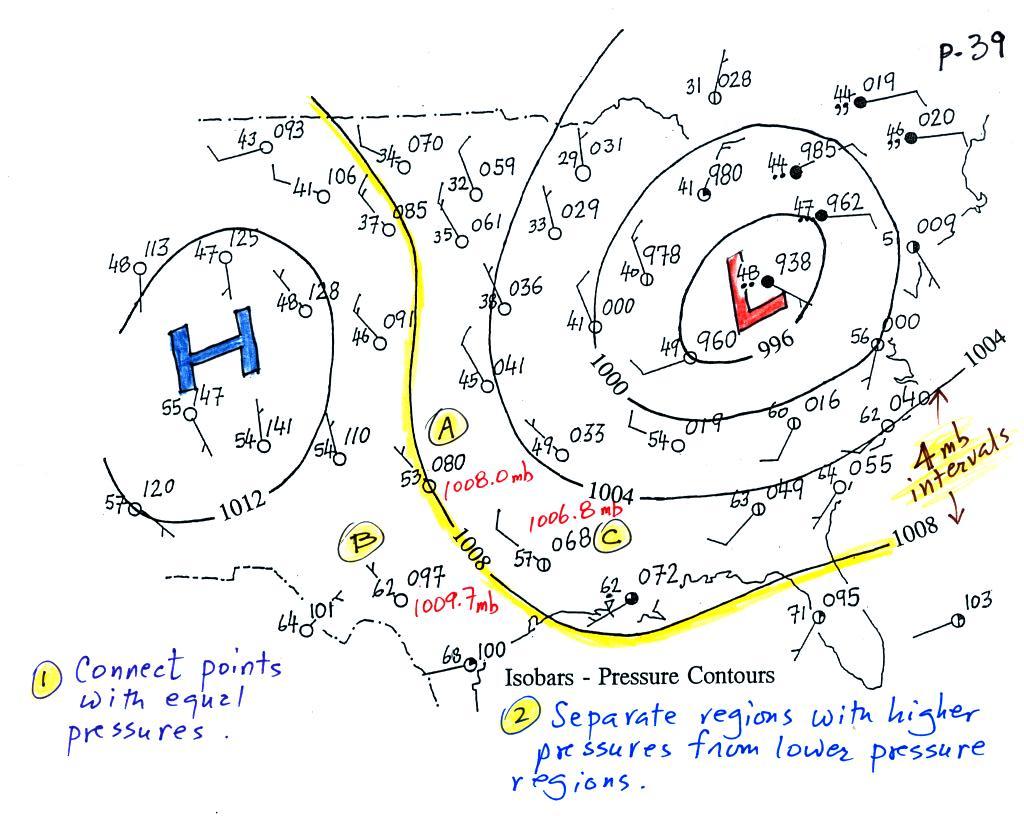
Now the same data with isobars
drawn in. Again they
separate
regions with pressure higher than a particular value from regions with
pressures lower than that value.
The isobars also enclose areas of high pressure and low pressure.
Isobars are generally drawn at 4 mb intervals (starting with a base
value of 1000 mb). Isobars
also connect points on the map
with the same pressure. The 1008 mb isobar (highlighted in
yellow) passes through a city at Point
A where the pressure is exactly
1008.0 mb. Most of the time the isobar
will pass between two
cities. The 1008 mb isobar passes between cities with
pressures
of 1009.7 mb at Point B and
1006.8 mb at Point C.
You would
expect to find 1008 mb somewhere in between
those two cites, that is where the 1008 mb isobar goes.
The pressure pattern is not as predictable as the isotherm
map. Low pressure is found on the eastern half of this map and
high pressure in the west. The pattern could just as easily have
been reversed.
This
site (from the American Meteorological Society) first shows surface
weather observations by themselves (plotted using the station model
notation) and then an analysis of the surface data like what we've just
looked at. There are links below each of the maps that will show
you current surface weather data.
Here's a little practice (this figure wasn't
shown in class).
Is this the 1000, 1002, 1004,
1006, or 1008 mb isobar? (you'll find the answer at the end of today's
notes)
Now we'll look at what you can learn about the weather once you've
drawn in some isobars and mapped out the pressure pattern.
1.
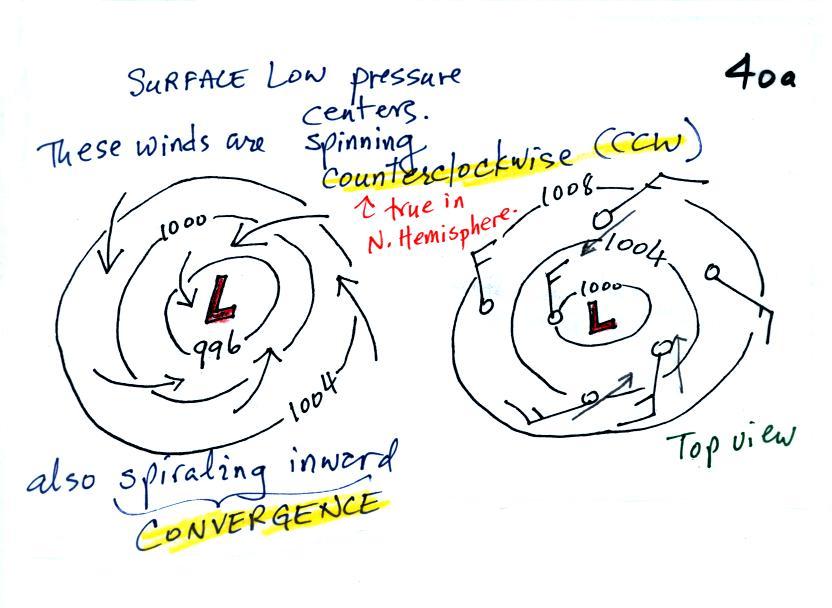
We'll start with the large nearly circular centers of High and Low
pressure. Low pressure is drawn below. These figures are
more neatly drawn versions of what we did in class.
Air will start moving
toward low
pressure (like a rock sitting on a hillside that starts to roll
downhill), then something called the Coriolis force will cause
the
wind to start to spin (we'll learn more about the Coriolis force later
in the semester). In the northern hemisphere winds spin in a
counterclockwise (CCW) direction
around surface
low pressure
centers. The winds also spiral inward toward the center of the
low, this is called convergence. [winds spin clockwise around low
pressure centers in the southern hemisphere but still spiral inward,
don't worry about the southern hemisphere until later in the semester]
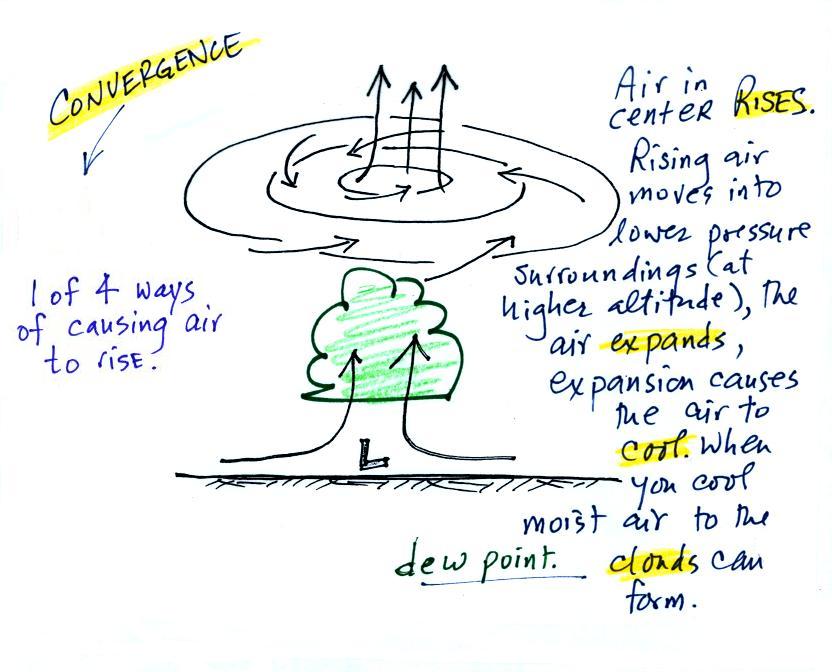
When the converging air reaches the
center of the low it starts to rise.
Rising air expands (because it is moving into lower pressure
surroundings at higher altitude), the expansion causes it to
cool. If the air is moist
and it is cooled enough (to or below the dew point temperature) clouds
will form and may then begin to rain or snow. Convergence is 1 of 4 ways of causing air
to rise (we'll learn what the rest are soon, and, actually, you
already know what one of them is - warm air rises, that's called
convection).
You
often
see
cloudy
skies
and
stormy
weather
associated with surface low pressure.
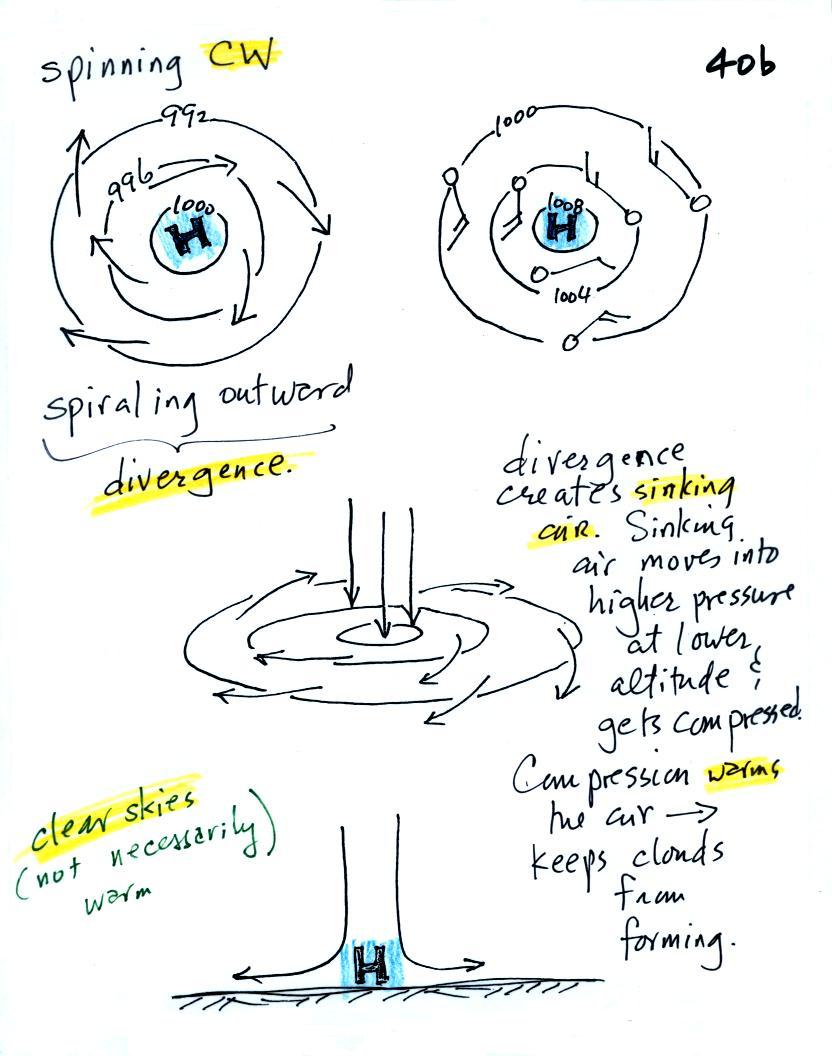
Everything is pretty much the exact opposite in the case of surface
high pressure.
Winds
spin
clockwise
(counterclockwise
in
the
southern
hemisphere)
and spiral outward.
The
outward motion is called divergence.
Air sinks in the center of
surface high pressure to
replace the diverging air. The sinking air is compressed and
warms. This keeps clouds from forming so clear
skies are normally found with high pressure.
Clear skies doesn't necessarily mean warm weather, strong surface high
pressure often forms when
the air is very cold.
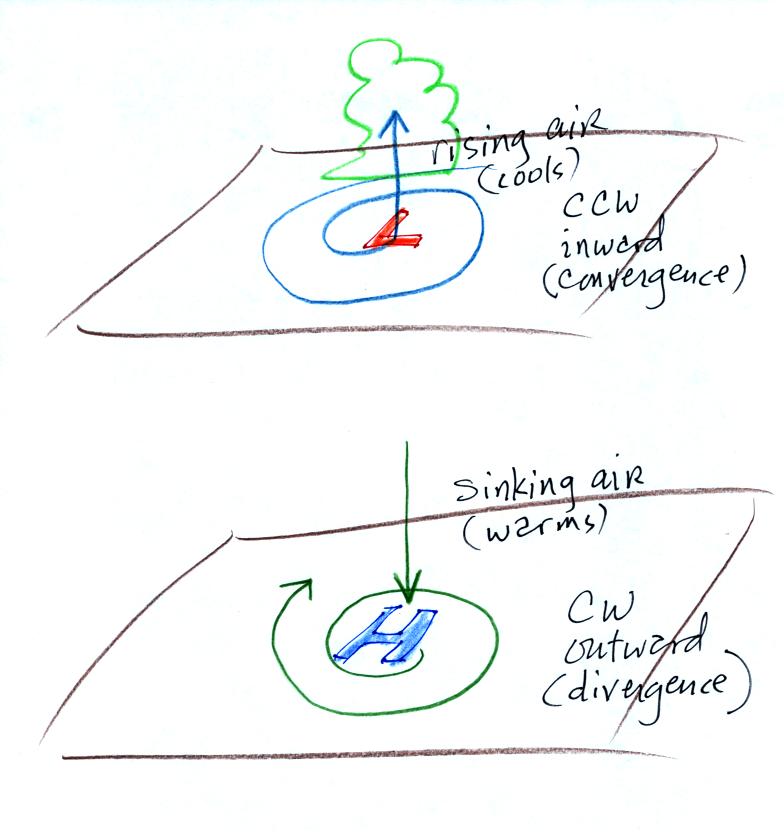
Here's a picture summarizing what we've learned so far. It's
a slightly different view of wind motions around surface highs and low
and wasn't
shown in class.
Here's another way of trying to
understand why warm air rises and cold air sinks - Archimedes
Law. It's a perhaps simpler way of understanding the
topics. A bottle of water can help you to visualize the law.
A gallon of
water weighs about 8 pounds (lbs). I would want to carry a gallon
of water on a hike unless I really thought I would need it.
If you submerge the gallon jug of water in a swimming pool, the
jug
becomes, for all intents and purposes, weightless. That seems
kind of amazing. Archimedes'
Law (see figure below, from p. 53a in the photocopied ClassNotes)
explains why this is true.

Archimedes first of all tells you
that the surrounding fluid will exert an upward pointing bouyant force
on the submerged water bottle. That's why the submerged jug can
become weightless.
Archimedes law also tells you how to figure
out how strong the bouyant force will be. In this
case the 1 gallon bottle will displace 1 gallon of
pool water. One
gallon of pool
water weighs 8 pounds. The upward bouyant force will be 8 pounds,
the same as the downward force. The two
forces are equal and opposite.
What Archimedes law doesn't really tell you is what causes the
upward
bouyant
force. If you're really on top of this material you will
recognize that it is really
just another name for the
pressure difference force that we covered on Wednesday (higher pressure
pushing
up on the bottle and low pressure at the top pushing down, resulting in
a net upward force).
Now we imagine pouring out all the water and filling the 1 gallon
jug
with air. Air is about 1000 times less dense than water; compared
to water, the jug
will weigh practically nothing.

If you
submerge the jug of air in a
pool
it will displace 1 gallon of
water
and experience an 8 pound upward bouyant force again. Since there
is no downward force the jug will float.
One gallon of sand (which is about 1.5 times denser than water)
jug weighs 12 pounds (I checked this out because I like to try to give
you accurate information).
The jug of sand will sink because
the downward force is greater
than
the upward force.
You can sum
all
of
this up by saying anything that is less dense
than
water will float in water, anything that is more dense than water will
sink in water.
Most types of wood will float. Most rocks won't
(pumice for example often floats).
The same reasoning applies to air in the atmosphere.
Air that is less dense (warmer)
than the air around it will
rise.
Air that is more dense (colder) than the air around it will sink.
Here's a little more
information
about
Archimedes that I didn't mention in
class.
There's a colorful demonstration that shows how small differences
in density
can determine whether an object floats or sinks.

A can of regular Pepsi (actually it
was Cherry Pepsi) was
placed in a beaker of water.
The
can
sank. A can of Diet Pepsi on the other hand floated.
Both cans are made of aluminum which has a density almost three
times
higher than water; aluminum by itself would sink. The drink
itself is largely water. The
regular soda also has a lot of high-fructose
corn
syrup, the diet soda
doesn't. The mixture of water and corn syrup has a density
greater than plain
water. There is also a little air (or perhaps carbon dioxide gas)
in each can.
The average density of the can of regular soda (water & corn
syrup
+
aluminum + air) ends up being slightly greater than the density of
water. The average density of the can of diet soda (water +
aluminum + air) is slightly less than the density of water.
I sometimes repeat the "demonstration" with a can of Pabst Blue
Ribbon
beer. This also floats because the beer doesn't contain any corn
syrup
(I don't think).
In some respects people in swimming pools are like cans of regular
and
diet soda. Some people float (they're a little less dense than
water), other people sink (slightly more dense than water).

Finally here's the answer to the question found earlier in today's
notes.
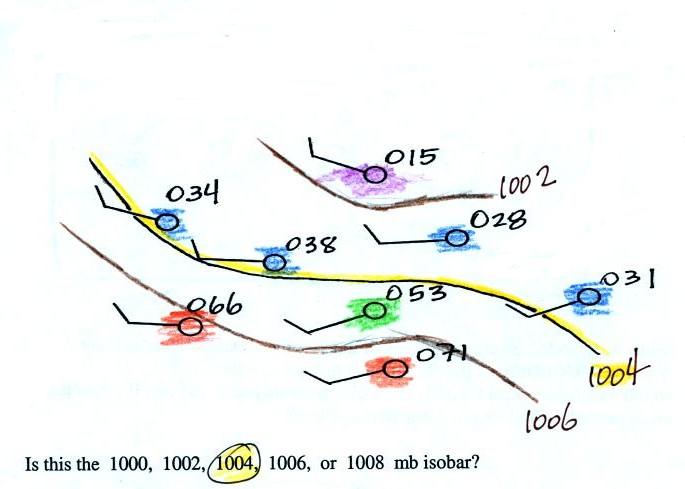
Pressures lower than 1002 mb are
colored purple. Pressures
between 1002 and 1004 mb are blue. Pressures between 1004 and
1006 mb are green and pressures greater than 1006 mb are red. The
isobar appearing in the question is highlighted yellow and is the 1004
mb isobar. The 1002 mb and 1006 mb isobars have also been drawn
in (because isobars are drawn at 4 mb intervals starting at 1000 mb,
1002 mb and 1006 mb isobars wouldn't normally be drawn on a map)