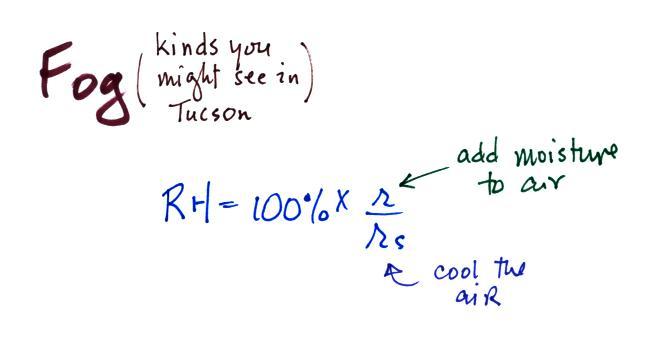One of the ways of measuring humidity is to use a sling
(swing might be more descriptive) psychrometer.
A sling
psychrometer consists of two thermometers mounted
side by side. One is an ordinary thermometer, the other is
covered with a wet piece of cloth (normally moisted with distilled
water). To
make a humidity measurement you swing the psychrometer around for a
minute or two and then read the temperatures from the two
thermometers. Measurements of the air temperature (dry bulb) and
the dry-wet thermometer temperature difference (wet bulb depression)
can be
used to determine relative humidity and dew point.

The evaporation is shown as blue arrows because this will cool the
thermometer. The same thing would happen if you were to step out
of a swimming pool on a warm dry day, you would feel cold.
Evaporative coolers (swamp coolers) work well (sometimes too well) on
days like this.
The figure at upper left also shows one arrow of condensation.
The amount or rate of condensation
depends on how much water vapor is
in the air surrounding the thermometer. In this case (low
relative humidity) there isn't much water vapor and not much
condensation. The
condensation arrow is orange because the condensation will release
latent heat and warm the thermometer.
Because there is more evaporation (4 arrows) than condensation (1
arrow) the wet bulb thermometer will drop.
The wet thermometer will cool but it won't cool indefinitely. We
imagine that the wet bulb thermometer
has cooled to 60 F. Because the wet piece of cloth is cooler,
the water is evaporating more slowly. The wet bulb thermometer
has cooled to
a temperature where the evaporation and condensation are in
balance. The thermometer won't cool any further.
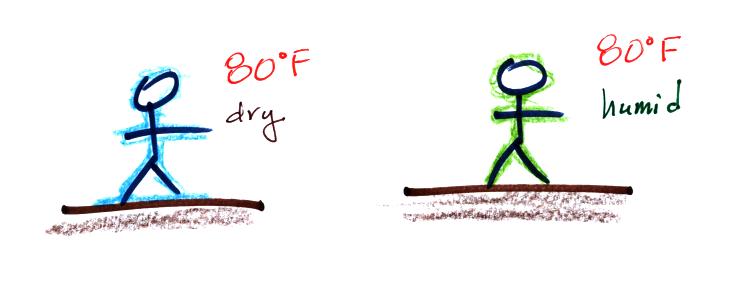
You
would measure a large difference (20 F) between the dry and wet bulb
thermometers on a day like this when the air is relatively dry.
The air temperature is the same in this
example, but there is more
water vapor in the air.
You wouldn't feel as cold if you stepped out of a pool and swamp
coolers wouldn't provide much cooling on a
warm humid day like this.
There are four arrows of evaporation (because the water temperature is
still 80 F just as it was in the previous example) and three arrows now
of
condensation (due to the increased amount of water vapor in the air
surrounding the thermometer). The wet bulb thermometer will cool
but won't get as
cold as in the previous example.
The wet bulb thermometer might well only cool to 75 F. This might
be enough to lower the rate of evaporation (from 4 arrows to 3 arrows)
enough to bring it into
balance with the rate of condensation.
You would measure a small difference (5 F) between the dry and wet bulb
thermometers on a humid day like this.

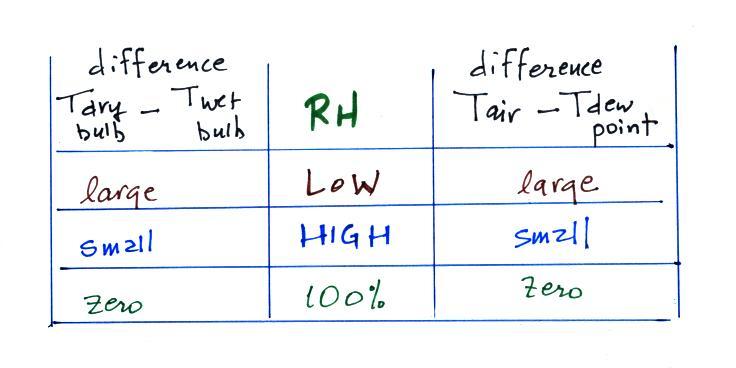
The chart below summarizes the relationship between relative humidity
and the Tdry - Twet difference. The chart also includes something
we mentioned a lecture or two ago, the
difference between air temperature and dew point temperature.
We learned
about wind chill earlier in the course.
A 40 F day with 30 MPH winds will feel colder (because of
increased transport of energy from your body by convection) than a 40 F
day
with no wind. The wind chill
temperature tells you how much colder it will feel.
Evaporative cooling will make you feel cold if you get
out of a swimming pool on an 80 F day with dry air.
You won't feel as cold if the air is humid. Sling
psychrometers make use of this to
measure relative humidity and
dew point.
Your body tries to stay cool by perspiring. You
would still feel
hot on
a hot dry day. The heat index
measures how much hotter you'd feel on a hot humid day. The
combination of heat and high humidity is a serious weather hazard
because it can cause heatstroke
(hyperthermia).
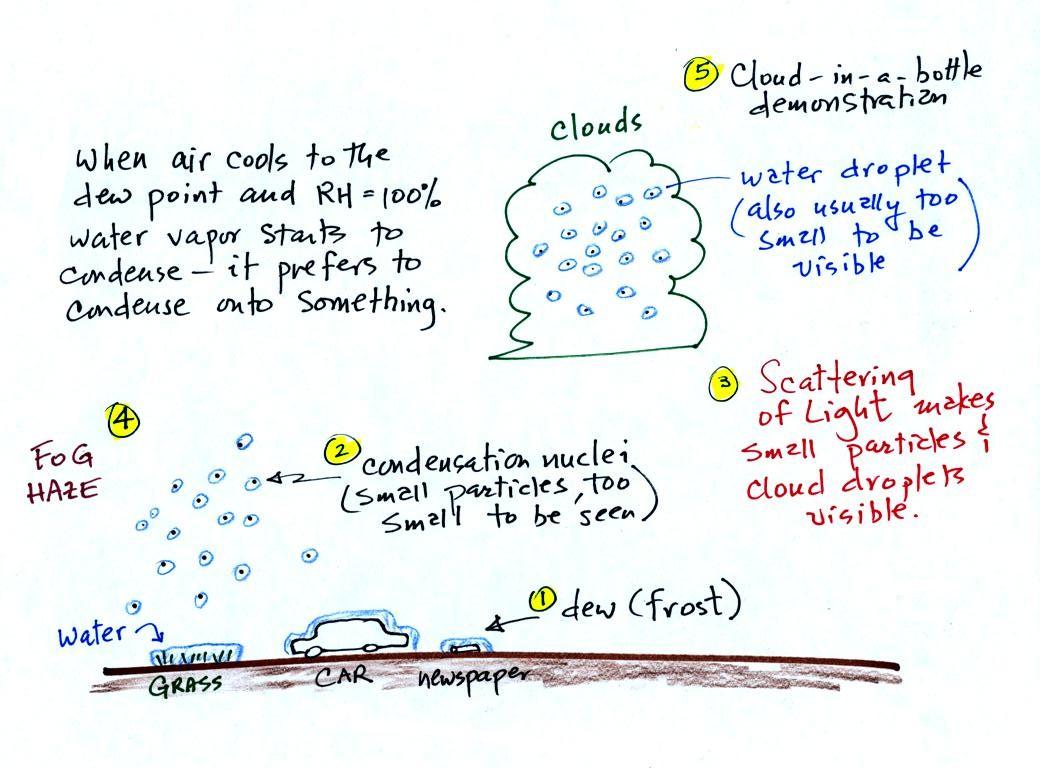
A variety of things can happen when you cool air to the dew point and
the relative humidity increases to 100%. Point 1
shows that when moist air next to the ground is cooled to
and
below the
dew point, water vapor condenses onto (or is deposited onto) the ground
or objects on the ground. This forms dew, frozen dew, and
frost.
Air above the ground can also be cooled to the dew point. When
that happens (Point 2 above) it is much easier for water vapor to
condense
onto very small particles in the air (condensation nuclei) rather than
just forming a small droplet of pure
water. The small water
droplets that form are themselves usually too small to be seen with the
naked eye. We can tell they are present (Point 3) because they
either scatter (haze or fog) or reflect (clouds) sunlight.
We'll start by looking at the
different conditions that can lead to the formation of dew and frost.

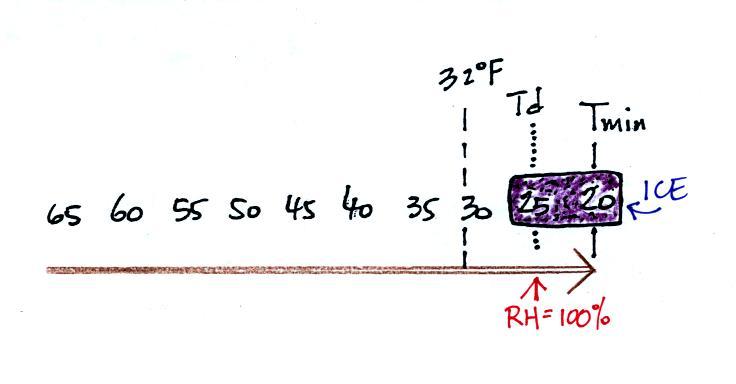
It might be a little hard to figure out what is being
illustrated
here. Point 1 is sometime in the early evening when the
temperature of the air at ground level is 65. During the course
of the coming night the air will cool to 35 F (the nighttime minimum
temperature, Tmin. Sometime during the night, at Point 2, the air
temperature reaches 40
F, the dew point. The relative humidity reaches 100% and water
vapor
begins to condense onto the ground. This continues as the air
cools to Tmin. You would find your newspaper
and your car covered with dew
the next morning.
This night is similar except that the nighttime
minimum
temperature drops below freezing. Dew forms and first covers
everything on the ground with water. Then the water freezes and
turns to ice. This isn't frost, rather
frozen dew. Frozen dew is
often thicker and harder to scrape off
your car windshield than frost.
Now the dew point and the nighttime minimum temperature are both
below
freezing. When the RH reaches 100% water vapor turns directly to
ice (deposition). This is frost.
What happens on this night? Because the nighttime minimum
temperature never reaches the dew point, the RH never reaches
100%. Nothing would
happen.
As mentioned earlier, when the
relative humidity in air above the ground (and away from objects on the
ground) reaches 100%, water vapor will condense onto small particles
called condensation nuclei.

It would be much harder for the
water
vapor to just condense and form small droplets of pure water.
This is because very small droplets have an unusually high rate of
evaporation. This is known at the curvature effect.
Saturated air (RH=100%) is not able to supply enough condensation to
offset the high rate of evaporation. If a small droplet suddenly
forms it will quickly evaporate away.
The figure below shows 4 droplets of varying radii. Note that the
rates of condensation (3 arrows) are equal in all 4 cases. The
rate of condensation depends on the amount of moisture in the air
surrounding each drop and that is the same for each droplet.

Once the droplet grows to a certain size as in 3, the rate of
evaporation has decreased to a point where it is balanced by an equal
amount of condensation. The rate of evaporation won't decrease
further if the droplet grows beyond this size. Droplets 3 and 4
have the same rates of evaporation. Both droplets are in
equilibrium with their surroundings (equal rates of evaporation and
condensation).
One way of avoiding the difficult shown above is for water vapor to
condense onto a small particle of some kind.

Small droplets can also form on CCN particles that dissolve. The
rate of evaporation from the resulting water solution is less than that
of pure water. This is known as the solute effect.
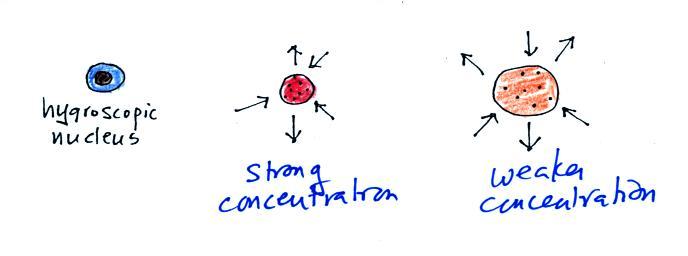
Water vapor has condensed onto a CCN particle at left in the figure
above. In the middle picture the CCN particle has dissolved and
formed a solution with strong concentration. This reduces the
rate of evaporation. Since condensation exceeds evaporation the
droplet will grow. Without the solute effect, a drop this small
would have a high rate of evaporation that would exceed the rate of
condensation and the droplet would evaporate.
Eventually the solution concentration weakens enough that it no longer
affects the evaporation rate. In the right picture the rates of
evaporation and condensation are equal and the droplet is in
equilibrium with its surroundings.
Because of the solute effect, it is possible for droplets to form when
the relative humidity is less than 100%. Condensation nuclei that
allow this to happen are called hygroscopic nuclei. This is
illustrated in the figure below. A small droplet has
formed. Because the resulting solution concentration is strong
there are only 2 arrows of evaporation. The droplet is in
equilibrium with surroundings that are only able to supply 2 arrows of
condensation (2 arrows of condensation in this figure compared with 3
arrows in the previous figures implies the RH is less than 100%).
In the classroom version of this course we show a short video that
demonstrates how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of
glass.
At the start of the demonstration
(left figure above) small grains
of
salt (soluble in water) were
placed on a platform in a petri dish
containing water. Some small spheres of glass (insoluble) were
placed in the
same
dish. After about 1 hour small drops of water form around
each
of the grains of salt but not the glass grains (shown above at
right).
In
humid parts of the US, water will condense onto the grains of
salt
in a salt shaker causing them to stick together. Grains of rice
apparently absorb moisture which keeps this from happening and allows
the salt to flow
freely out of the shaker when needed.
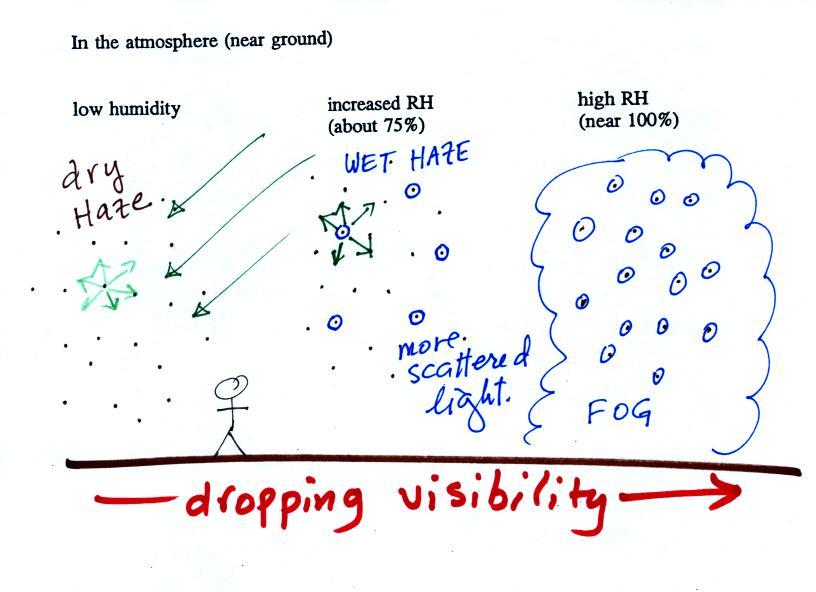
This figure shows
how
cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even
though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter
sunlight. When you look at the sky you see the deep blue color
caused by scattering of sunlight by air molecules mixed together with
some white
sunlight scattered by the condensation nuclei. This changes
the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze."
The middle picture shows what happens when you drive from the dry
southwestern part of the US into the humid
southeastern US. One of the first things you would notice is the
hazier
appearance of the air and a decrease in visibility. Because the
relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze."
Finally when the relative humidity increases to 100% fog
forms.
Fog can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation nuclei.
Fog can be
produced in a variety of ways. To produce fog you
first need to
increase the relative humidity (RH) to
100%
You can do this either by cooling the air (radiation fog) or
adding
moisture to
and saturating the air (evaporation or steam fog). Both will
increase the ratio in the RH formula
above.
Probably the most common type of fog in Tucson is radiation fog.
The ground cools during the night by emitting IR radiation (left figure
below). The ground cools most rapidly and gets coldest when the
skies are free of
clouds and the air is dry (except for a thin layer next to the
ground.
Air in contact with the ground cools and radiation fog can form
(right
figure above). Because the fog cloud is colder than the air right
above, this is a stable situation. The fog clouds "hugs" the
ground.
Radiation fog is sometimes called valley fog.
The cold dense foggy air will move downhill and fill low lying
areas. Because the fog reflects sunlight, it is often
difficult for the sun to warm the air
and dissipate thick clouds of valley fog.
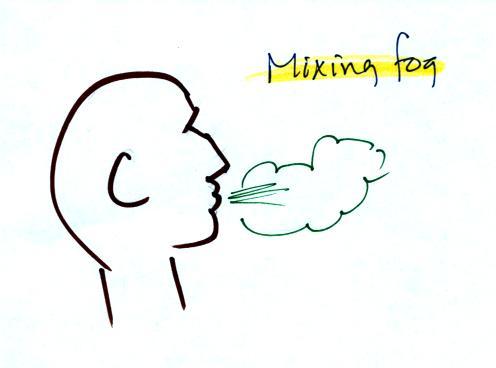
Steam fog or evaporation fog (also sometimes known as mixing fog) is
commonly observed on
cold mornings over the relatively warm water in a swimming pool.
Water evaporating from the pool
saturates the cold air above. Because the fog cloud is warmer
than the cold surrounding air, the fog clouds float upward.
When you "see your breath" on a cold day
you're seeing mixing fog. Warm moist air from your mouth mixes
with the colder air outside. The mixture is saturated and a fog
cloud forms.
Here's another demonstration from the classroom version of this course
that puts together many of the
concepts we have been covering. Cooling
air and
changing relative humidity, condensation nuclei, and scattering of
light are all involved in this demonstration.
We used a strong, thick-walled, 4 liter flask (vaccum flasks
like this are designed to not implode when all of the air is pumped out
of them, they aren't designed to not explode when pressurized).
There
was a little
water in the bottom of the flask to moisten the air in the flask.
Next we pressurized the air in the flask with a bicycle pump. At
some point the
pressure blows the cork out of the top of the flask.
The air in
the flask expands outward and cools. This sudden cooling
increases the
relative humidity of the moist air in the flask to 100% ( probably more
than 100% momentarily ) and water vapor condenses onto cloud
condensation nuclei in
the air. A faint cloud became visible at this point. The
cloud droplets are too small to be seen with the human eye. You
can see the cloud because the water droplets scatter light.

The demonstration was repeated an
additional time with one
small
change. A burning match was dropped into the
bottle. The smoke from the match added lots of very small
particles, condensation nuclei, to the air in the flask. The
cloud that formed
this time was quite a bit "thicker" and much easier to see.
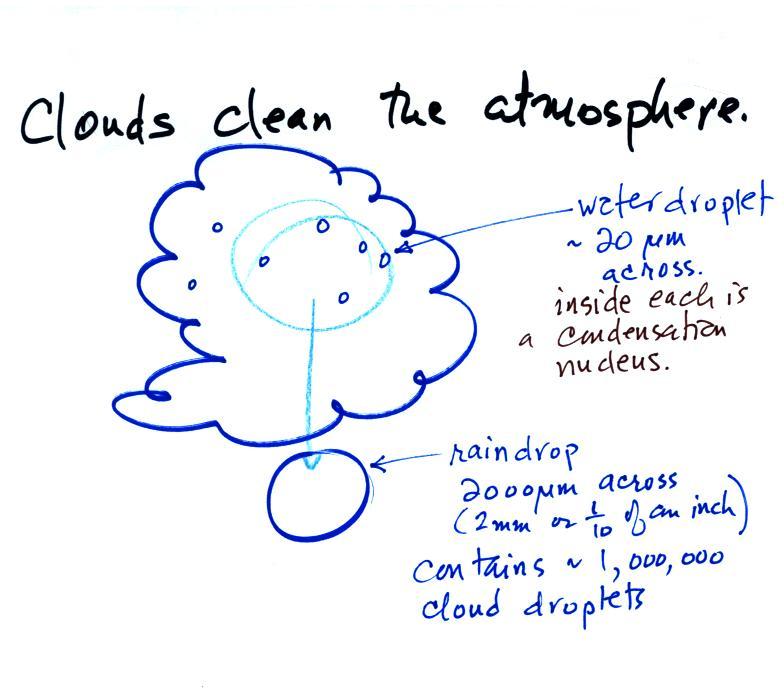
Clouds are one of the best ways of cleaning the
atmosphere
(cloud
droplets form on particles, the droplets "clump" together to form a
raindrop, and the raindrop carries the particles to the ground).
A raindrop can contain 1 million cloud droplets so a single raindrop
can remove a lot of particles from the air. You may have noticed
how clear the air seems the day after a rainstorm; distant mountains
are crystal clear and the sky has a deep blue color. Gaseous
pollutants can dissolve in the water droplets and be carried to
the ground by rainfall also.
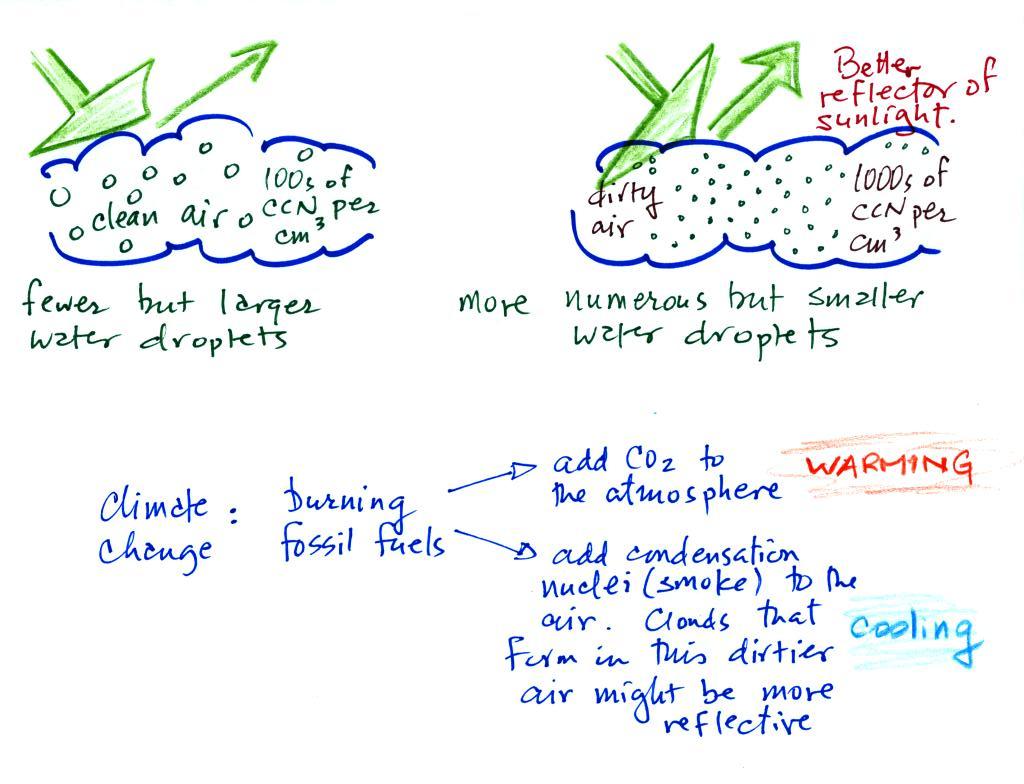
A cloud that forms in dirty air is composed of a large
number of small droplets (right figure above). This cloud is more
reflective
than a cloud that forms in clean air, that is composed of a smaller
number of larger
droplets (left figure).
Just like in the cloud-in-a-bottle demonstration, the cloud that was
created when the air was full of smoke particles was much more visible
than the cloud made with cleaner air.
This is has implications for climate change.
Combustion of fossil fuels adds carbon dioxide to the atmosphere.
There is concern that increasing carbon dioxide concentrations will
enhance the greenhouse effect and cause global warming.
Combustion also adds condensation nuclei to the atmosphere (just like
the burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form, might make
the clouds more reflective, and might cause cooling. There is
still quite a bit of uncertainty about how clouds might change and how
this
might affect climate (remember too that clouds are good absorbers of IR
radiation).