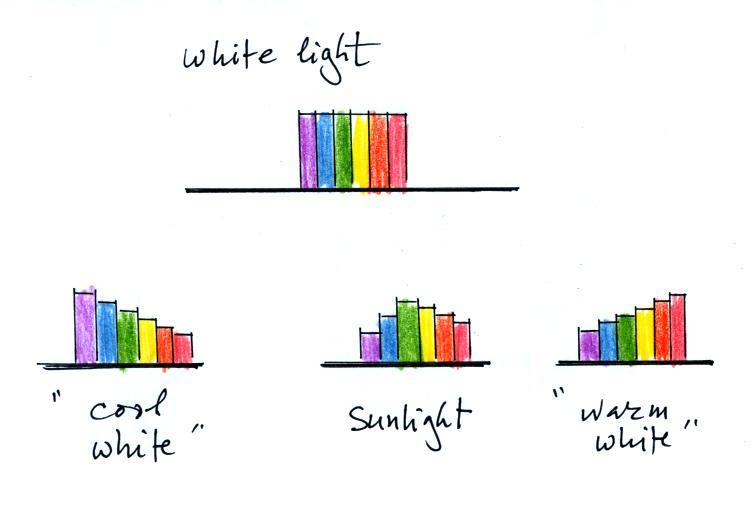
Warm white contains more of the
longer wavelength colors. Cool white contains more of the shorter
wavelengths.
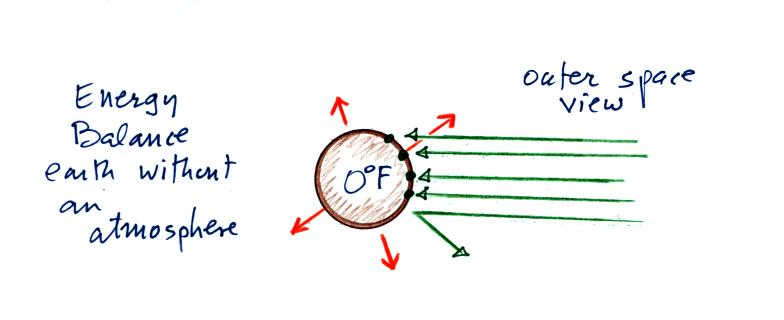
We'll start back where we left off last Monday with the
outer space view of radiative equilibrium on the earth without an
atmosphere. The important thing to note is that the earth is
absorbing and emitting the same amount of energy (4 arrows absorbed
balanced by 4 arrows emitted). The arrow of reflected sunlight
doesn't any role at all.
We will be moving from outer space to the earth's surface (the next two figures below).
Don't let the fact that there are
4 arrows are
being absorbed and
emitted in the figure above and
2 arrows absorbed and emitted in the bottom figure below
2 arrows absorbed and emitted in the bottom figure below
bother you. The important
thing is that there are equal
amounts being absorbed and emitted in both cases.
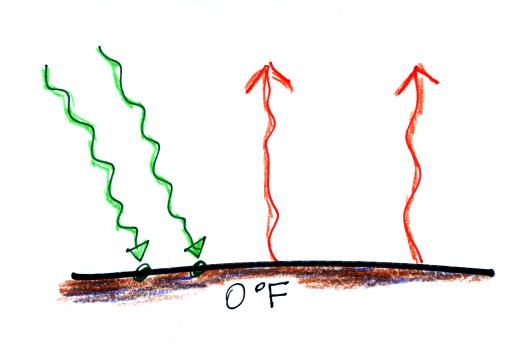
The reason for only using two
arrows in this picture is to keep the picture as simple as possible
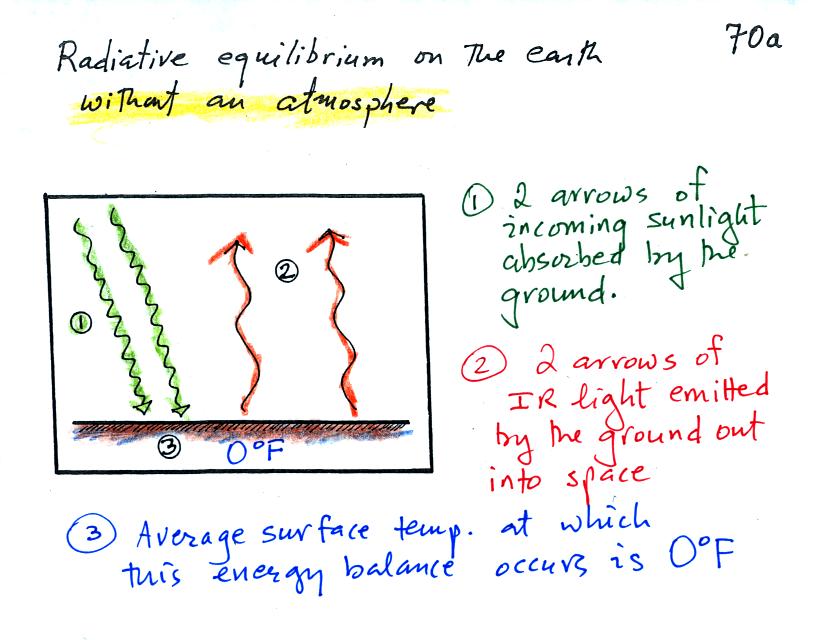
Here's the same picture with some
more information added (p. 70a in the photocopied ClassNotes).
This
represents energy balance on the earth without an atmosphere.
The next step is to add the atmosphere.
We will study a simplified version of radiative equilibrium just so you can identify and understand the various parts of the picture. Keep an eye out for the greenhouse effect. Here's a cleaned up version of what we ended up with in class (I added a little information at the bottom of the picture.
The next step is to add the atmosphere.
We will study a simplified version of radiative equilibrium just so you can identify and understand the various parts of the picture. Keep an eye out for the greenhouse effect. Here's a cleaned up version of what we ended up with in class (I added a little information at the bottom of the picture.
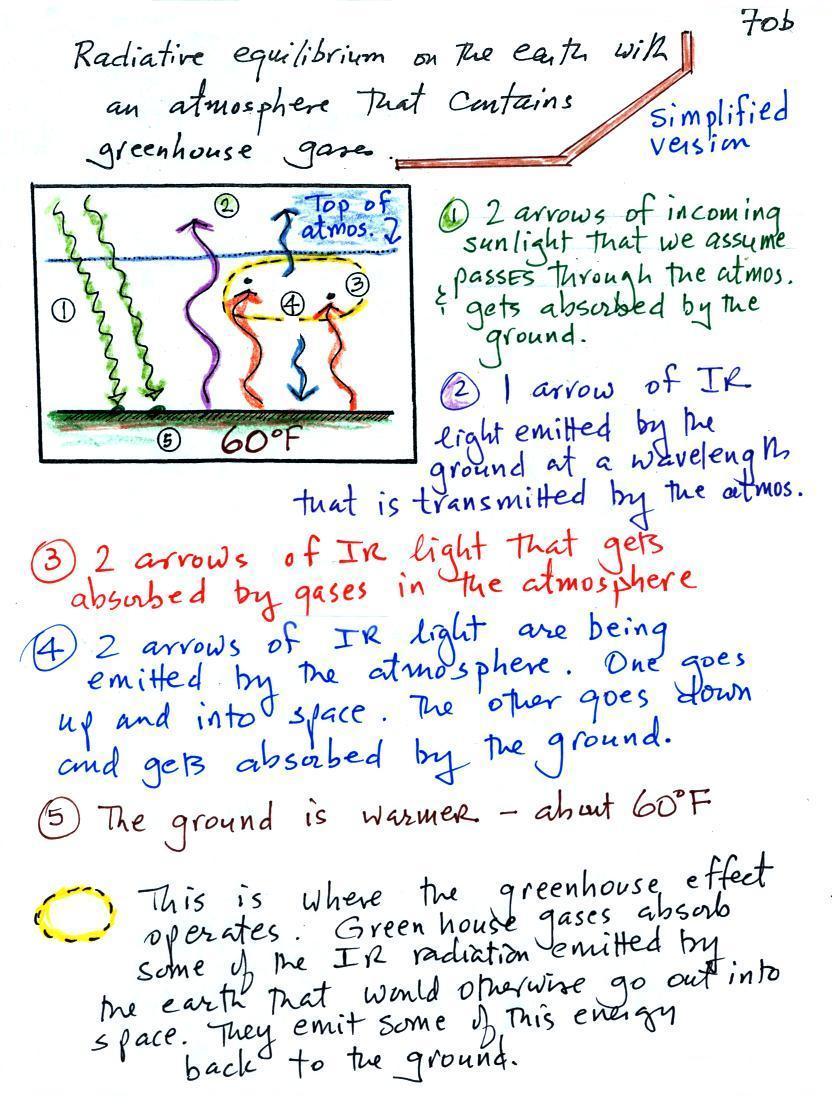
It would be hard to sort through
and try to understand all of this
if you weren't in
class
(difficult even if you were in class). So below we
will go through it again step by step (which you are free to skip over
if you wish). This is a more
detailed version than was done in class. Caution:
some of the colors below are different
from those used in class.
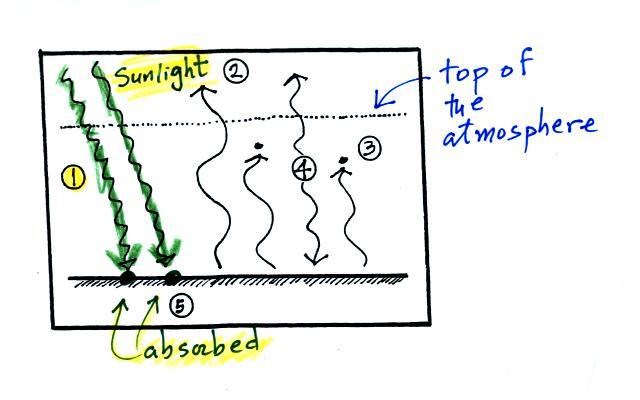
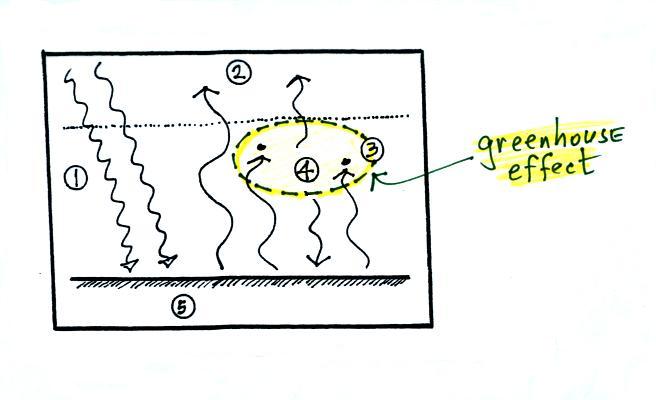
1. In this
picture we see the two
rays of incoming sunlight that
pass through the atmosphere, reach the ground, and are absorbed.
100% of the incoming sunlight is transmitted by the atmosphere.
This wouldn't be too bad of an assumption if sunlight were just visible
light. But it is not, sunlight is about half IR light and some of
that
is going to be absorbed. But we won't worry about that at this
point.
The ground is emitting
a total of 3 arrows of IR radiation. At this point that might
seem like a problem. How can the earth emit 3 arrows when it is
absorbing only 2. We'll see how this can happen in a second.
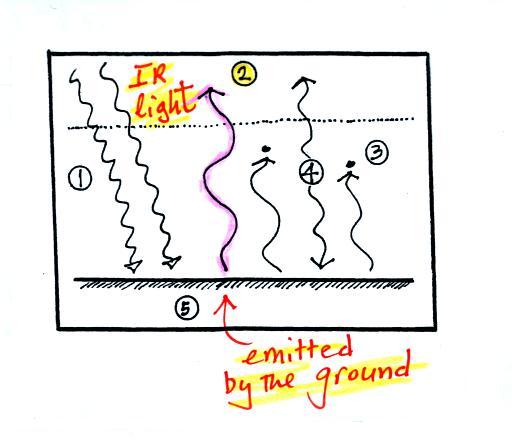

2. One
of
these
(the
pink
or
purple
arrow
above)
is
emitted
by
the
ground
at
a
wavelength
that
is not absorbed
by greenhouse gases in the atmosphere (probably around 10
micrometers, in the center of the "atmospheric window"). This
radiation passes through the atmosphere and goes out into space.
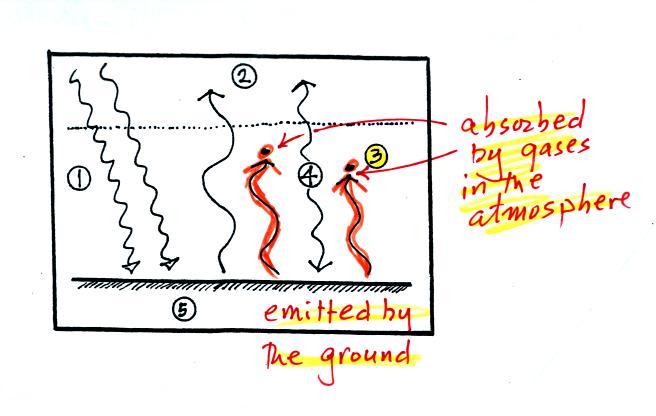
3. The other 2
units of IR radiation emitted by
the
ground are
absorbed by
greenhouse gases is the atmosphere.
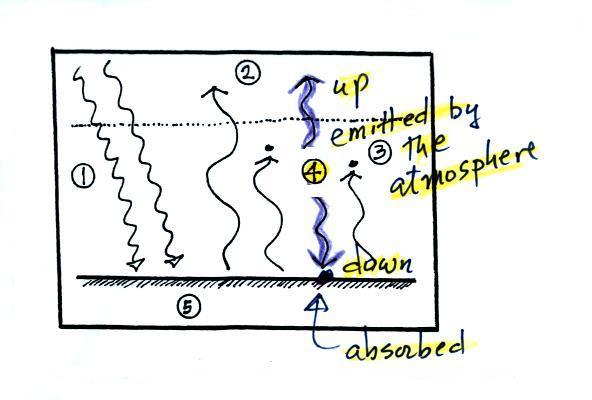
4. The
atmosphere is absorbing
2 units of radiation.
In order to be in radiative equilibrium, the atmosphere must also emit
2
units of radiation. That's shown above. 1
unit of IR radiation is sent upward into space, 1 unit is sent downward
to the ground where it is absorbed. This is probably the part of
the picture that most students have trouble visualizing (it isn't so
much that
they have trouble understanding that the atmosphere emits radiation but
that 1 arrow is emitted upward and another is emitted downward toward
the ground.
Before we go any further we will
check
to be sure that
every part
of this picture is in energy balance.
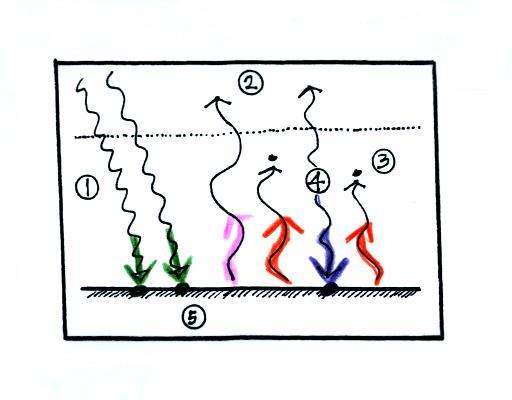
The ground is absorbing
3 units of energy (2 green
arrows of sunlight and one bluish arrow coming from the atmosphere) and
emitting
3
units of energy (one pink and two red arrows). So the ground is
in energy balance.
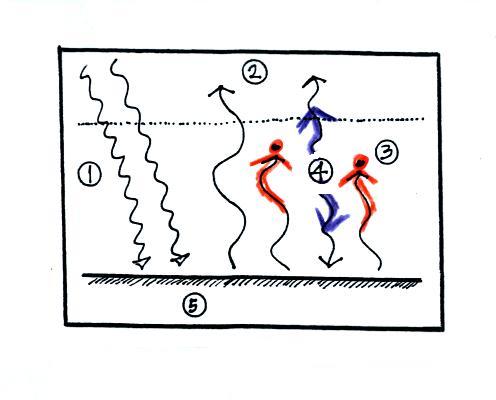
The atmosphere is
absorbing 2 units of energy (the 2
red arrows coming from the ground) and
emitting 2
units of
energy (the 2 blue arrows). One goes upward into space. The
downward arrow goes all the way
to the ground where it gets absorbed (it leaves the atmosphere and gets
absorbed by the ground). The atmosphere is in energy balance.
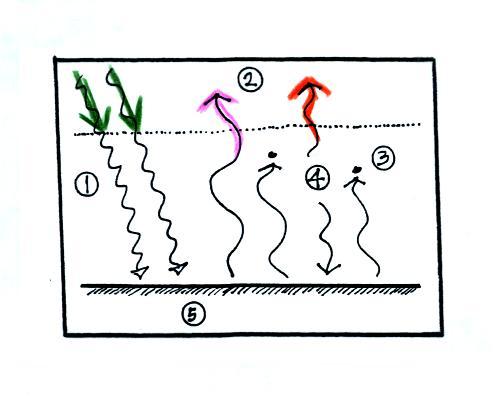
And we should check to
be sure equal amounts of energy
are arriving at and leaving the earth. 2 units of energy arrive
at the top of the atmosphere (green) from the sun after traveling
through space, 2 units
of
energy (pink and orange) leave the earth and head back out into
space. Energy balance here too.
The greenhouse effect
involves the absorption and
emission
of IR radiation by the atmosphere. Here's how you might put it
into words

Doesn't it make sense that if the ground is getting back some of the energy it would otherwise lose, the ground will end up being warmer. That's what the greenhouse effect does, it warms the earth's surface. The global annual average surface temperature is about 60 F on the earth with a greenhouse effect. It would be about 0 F without the greenhouse effect.
Here are a couple other ways of understanding why the greenhouse effect warms the earth.
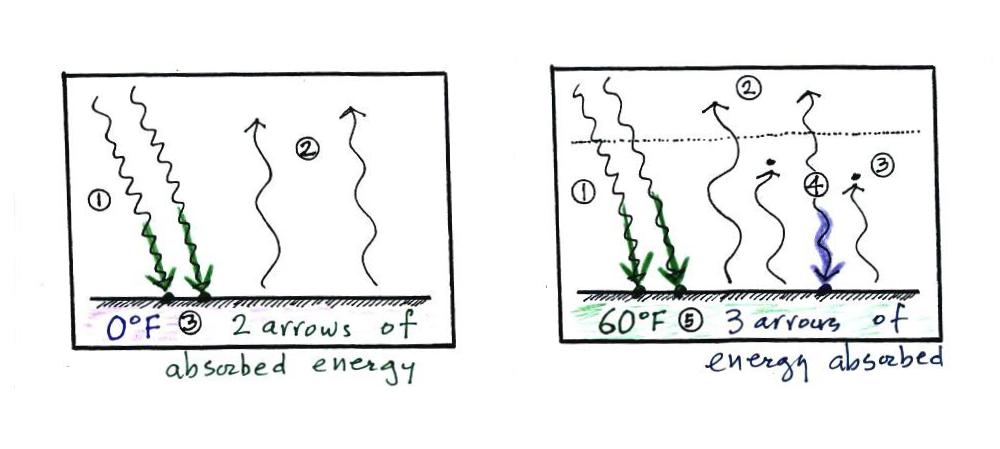
The picture at left is
the earth without an atmosphere (without a greenhouse effect). At
right the earth has an
atmosphere, one that contains greenhouse gases.
At left the ground
is getting 2 units of energy (from the sun). At right it is
getting three, two from the sun and one from the atmosphere (thanks to
the greenhouse effect).
Doesn't it seem
reasonable
that ground that absorbs 3 units of energy will be warmer than ground
that is only absorbing 2?
Here's another explanation of why the ground is warmer with a greenhouse effect than without.
Here's another explanation of why the ground is warmer with a greenhouse effect than without.
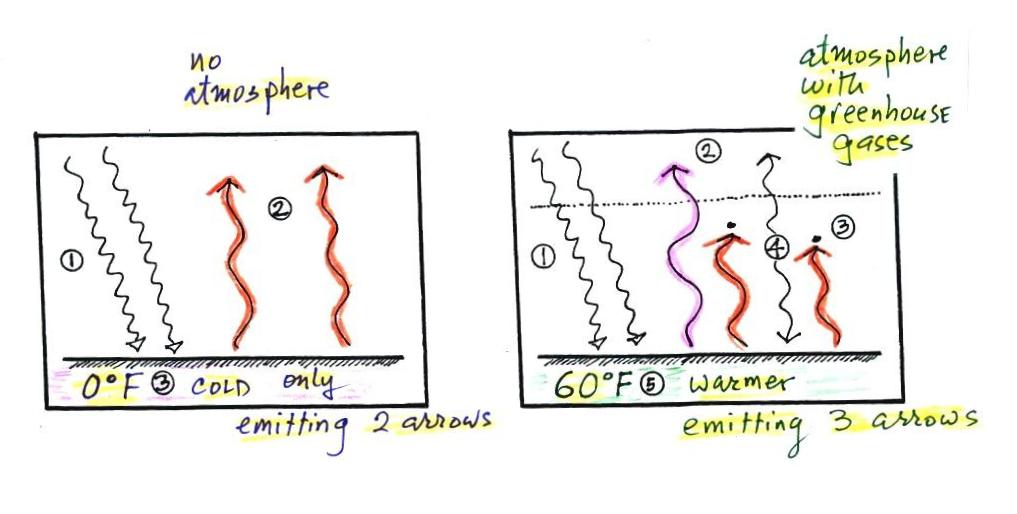
In our simplified explanation of the greenhouse effect we assumed that 100% of the sunlight arriving at the earth passed through the atmosphere and got absorbed at the ground. We will now look at how realistic that assumption is.
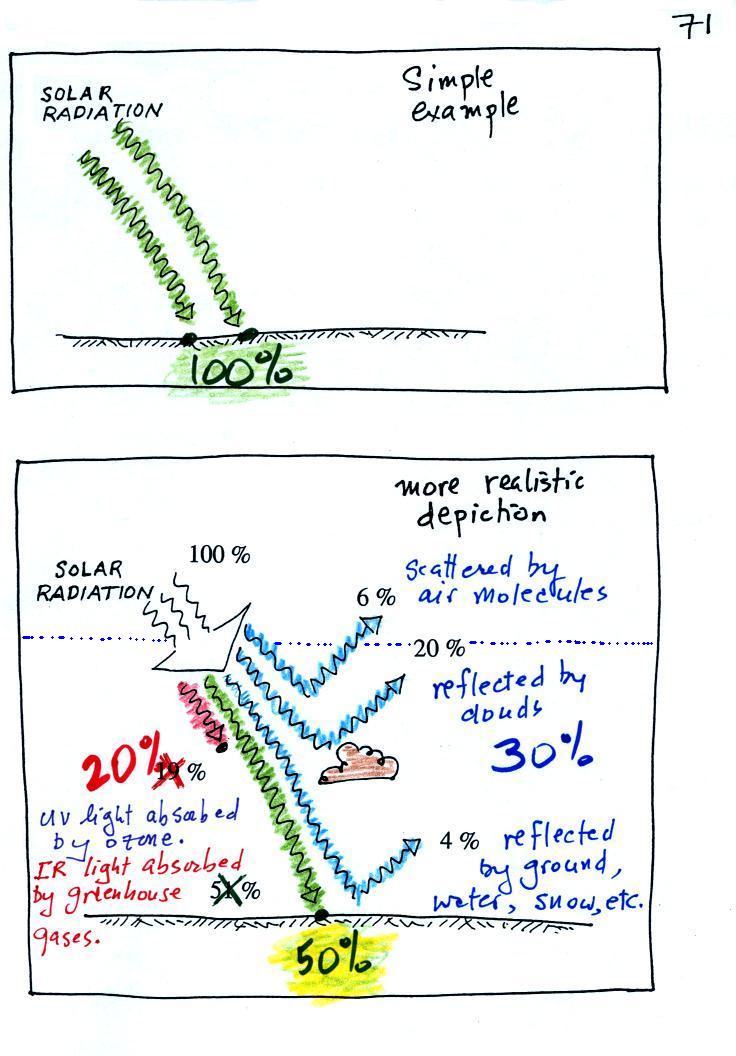
About 20% of the incoming sunlight is absorbed by gases in the atmosphere. Sunlight is a mixture of UV, VIS, and IR light. Ozone and oxygen will absorb a lot of the UV (UV makes up only 7% of sunlight) and greenhouse gases will absorb some of the IR radiation in sunlight (Roughly half of sunlight is IR light).
The remaining 30% of the incoming sunlight is reflected or scattered back into space (by the ground, clouds, even air molecules).
Students performing Experiment #3 will be measuring the amount of sunlight energy arriving at the ground. About 2 calories pass through a square centimeter per minute at the top of the atmosphere. Since about half of this arrives at the ground on average, students should expect to get an answer of about 1 calorie/cm2 min.
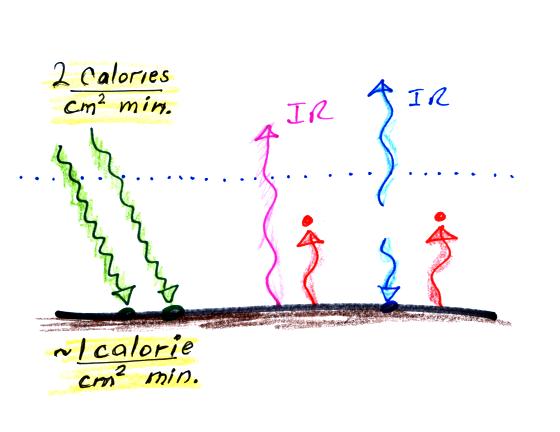
Now that we know a little bit more
about the fate of incoming sunlight we'll improve our simplified
illustration of the greenhouse effect somewhat. We'll make it a
little more realistic.
In this case we'll assume that 1 of the 2 incoming arrows of sunlight is absorbed in the atmosphere instead of passing through the atmosphere and being absorbed at the ground. The ground is still emitting 3 arrows of IR light. What would you need to add to this picture to bring it into energy balance? That was the first of two questions on an in-class Optional Assignment.
Start with the atmosphere. How many units does it need to emit. It's absorbing 3 units or energy and must, therefore, emit 3 arrows of radiation. How many should we draw going upward, how many go downward?
We'll next look at the ground. It is absorbing 1 unit of sunlight energy but emitting 3; it needs two more units of energy. Thus we should send 2 of the 3 arrows of radiation emitted by the atmosphere downward toward the ground.
We'll send the remaining arrow of energy emitted by the atmosphere upward and into space.
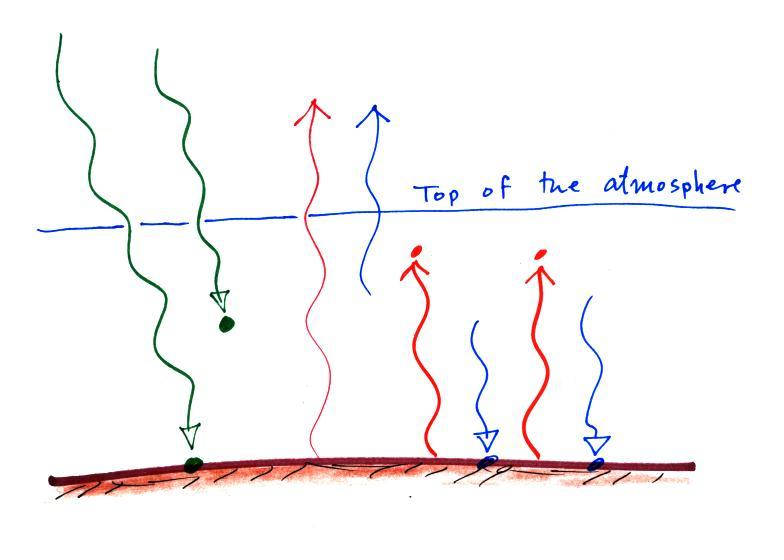
The atmosphere is emitting 3 arrows
of IR light. 1
goes upward and into space, the other two go downward and get absorbed
by the ground.
Next we will look at pretty realistic picture of energy balance on the earth (the bottom figure below). The simplified version that we just worked out earlier in the class is also shown for comparison (top figure).
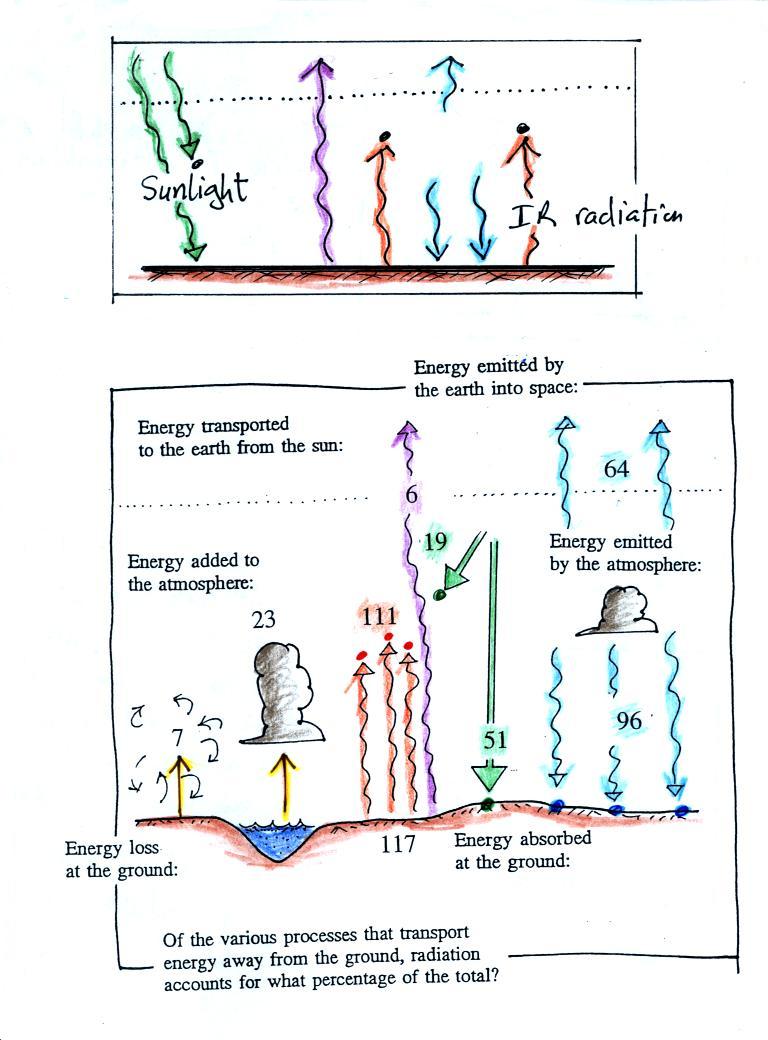
In the top figure (the simplified representation of energy balance) you should recognize the incoming sunlight (green), IR emitted by the ground that passes through the atmosphere (pink or purple), IR radiation emitted by the ground that is absorbed by greenhouse gases in the atmosphere (orange) and IR radiation emitted by the atmosphere (blue).
The lower part of the figure is pretty complicated. It would be difficult to start with this figure and find the greenhouse effect in it. That's why we used a simplied version. Once you understand the upper figure, you should be able to find and understand the corresponding parts in the lower figure (especially since I've tried to use the same colors for each of the corresponding parts).
Some of the incoming sunlight (51 units in green) reaches the ground and is absorbed. 19 units of sunlight are absorbed by gases in the atmosphere. The 30 units of reflected sunlight weren't included in the figure.
The ground emits a total of 117 units of IR light. Only 6 shine through the atmosphere and go into space. The remaining 111 units are absorbed by greenhouse gases. The atmosphere in turn emits energy upward into space (64 units) and downward toward the ground (96 units). Why are the amounts different? One reason might be that the lower atmosphere is warmer than the upper atmosphere (warm objects emit more energy than cold objects). Part of the explanation is probably also that there is more air in the bottom of the atmosphere (the air is denser) than near the top of the atmosphere.
Notice that conduction, convection, and latent heat energy transport (the 7 and 23 units on the left side of the figure) are needed to bring the overall energy budget into balance. The amount of energy transported by conduction, convection, and latent heat is small compared to what is transported in the form of EM radiation.
A couple more things to notice in the bottom figure.
(i) The ground emits more energy (117 units) than it gets from the sun (51 units). It is able to achieve energy balance because it also gets energy from the atmosphere (96 units).
(ii) The ground is actually receiving more energy from the atmosphere (96 units) than it gets from the sun (51 units)! Part of the reason for this is that the sun just shines for part of the day. We receive energy from the atmosphere 24 hours per day.
Here's another picture to test your understanding (the second question on the in-class assignment)
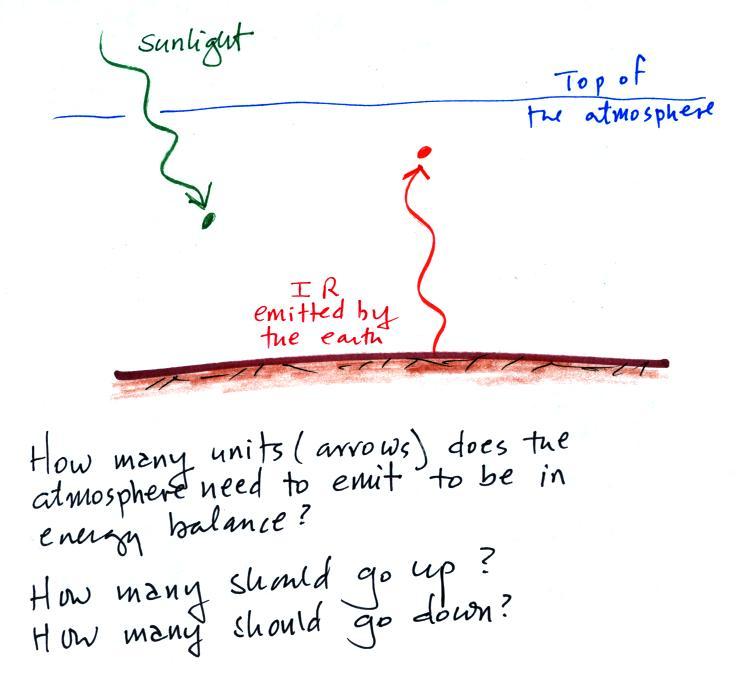
One unit of sunlight energy arrives at the earth and gets absorbed
by the atmosphere. It doesn't make it to the ground. The
ground is emitting one unit of energy that gets absorbed by the
atmosphere. You need to figure out what the atmosphere is doing
to bring this picture into energy balance. When you think you
have the answer click here.
Next we will look at pretty realistic picture of energy balance on the earth (the bottom figure below). The simplified version that we just worked out earlier in the class is also shown for comparison (top figure).

In the top figure (the simplified representation of energy balance) you should recognize the incoming sunlight (green), IR emitted by the ground that passes through the atmosphere (pink or purple), IR radiation emitted by the ground that is absorbed by greenhouse gases in the atmosphere (orange) and IR radiation emitted by the atmosphere (blue).
The lower part of the figure is pretty complicated. It would be difficult to start with this figure and find the greenhouse effect in it. That's why we used a simplied version. Once you understand the upper figure, you should be able to find and understand the corresponding parts in the lower figure (especially since I've tried to use the same colors for each of the corresponding parts).
Some of the incoming sunlight (51 units in green) reaches the ground and is absorbed. 19 units of sunlight are absorbed by gases in the atmosphere. The 30 units of reflected sunlight weren't included in the figure.
The ground emits a total of 117 units of IR light. Only 6 shine through the atmosphere and go into space. The remaining 111 units are absorbed by greenhouse gases. The atmosphere in turn emits energy upward into space (64 units) and downward toward the ground (96 units). Why are the amounts different? One reason might be that the lower atmosphere is warmer than the upper atmosphere (warm objects emit more energy than cold objects). Part of the explanation is probably also that there is more air in the bottom of the atmosphere (the air is denser) than near the top of the atmosphere.
Notice that conduction, convection, and latent heat energy transport (the 7 and 23 units on the left side of the figure) are needed to bring the overall energy budget into balance. The amount of energy transported by conduction, convection, and latent heat is small compared to what is transported in the form of EM radiation.
A couple more things to notice in the bottom figure.
(i) The ground emits more energy (117 units) than it gets from the sun (51 units). It is able to achieve energy balance because it also gets energy from the atmosphere (96 units).
(ii) The ground is actually receiving more energy from the atmosphere (96 units) than it gets from the sun (51 units)! Part of the reason for this is that the sun just shines for part of the day. We receive energy from the atmosphere 24 hours per day.
Here's another picture to test your understanding (the second question on the in-class assignment)
